P arkinson’s disease is the second most common neurodegenerative disease in the United States, affecting over 1 million individuals. While the motor symptoms that define the illness, such as tremor, rigidity, and postural imbalance, have received a great deal of attention, the nonmotor aspects of this condition are increasingly focused upon. Depression, one of the most prevalent nonmotor complications in Parkinson’s disease,
1 impacts as many as 50% of patients.
2,
3 The high prevalence of depression in Parkinson’s disease is of great clinical significance as it has a documented negative impact on quality of life, self-care, family relationships, and physical disability.
4 –
6 Moreover, depression has been linked to more severe and more rapidly progressive cognitive decline in Parkinson’s disease.
7 For example, difficulties with memory, attention, language, and executive functions have been frequently observed in Parkinson’s disease and are often exacerbated by depression, especially when that depression is pronounced.
8 –
11 Of these various cognitive abilities, memory and, to a lesser extent, language (i.e., verbal fluency and naming) appear to be the most severely affected by depression.
2,
3,
8 –
10,
12 While these cognitive changes are independently detrimental to the patient’s well-being
13 and have been found to predict nonresponse to psychopharmacological treatment in the aged,
14 –
16 they also further intensify the social, occupational, and functional impairment caused by both Parkinson’s disease and depression.
Despite the deleterious impact of depression in Parkinson’s disease, there are few well-designed treatment outcome studies that can guide clinical care. Moreover, few studies have investigated the impact of antidepressant treatment on the various aspects of cognitive functioning in Parkinson’s disease or the extent to which cognition affects antidepressant treatment response in this population.
17,
18 In an NIH-funded, randomized, double-blind trial of nortriptyline, paroxetine, and placebo for the treatment of depression in Parkinson’s disease, we recently demonstrated that nortriptyline was superior to placebo for the acute treatment of depression over an 8-week period
19 and that both active drugs were superior to placebo for the prevention of relapse over a 24-week period.
20 The purpose of this article is to describe the neuropsychological findings obtained after the acute and longer-term treatment of depression in Parkinson’s disease in this randomized, controlled trial and to detail cognitive predictors of treatment response.
METHODS
Overview
This randomized, controlled double-blind trial of nortriptyline, paroxetine, and placebo had two phases: an 8-week acute treatment phase and a 4-month extension phase. In the acute treatment phase, clinical response was defined
a priori as a 50% reduction in baseline to endpoint score on the Hamilton Depression Rating Scale (HAM-D).
21 However, patients were eligible to enter the extension phase of the study if they were rated at least minimally improved on the Clinical Global Impression Improvement Scale
22 (i.e., CGI-I rating of 1, 2, or 3) at the end of the acute treatment phase and wished to continue with blinded treatment. The results of neuropsychological testing obtained across both phases of this trial are detailed below. The study had the full approval of UMDNJ-Robert Wood Johnson Medical School institutional review board. All patients signed a statement of informed consent prior to the initiation of any study procedures.
Participants
Patients were recruited from the movement disorders clinic at Robert Wood Johnson Medical School, the New Jersey Chapter of the APDA, and local print media. All participants received free study medication and evaluation sessions and $20 for each completed study visit.
Fifty-two patients (27 men, 25 women; ages 35–80 years old) with a confirmed diagnosis of Parkinson’s disease based on research criteria
23 and a primary diagnosis of major depression or dysthymia based on the Structured Clinical Interview (SCID)
24 for the DSM-IV
25 were enrolled in the acute phase of the treatment trial. Patients with cognitive impairment (MMSE
26 score less than 26), “off time” greater than 50% of the day, any comorbid DSM-IV axis I diagnosis other than an anxiety disorder, or who had failed two or more adequate trials (dose and length) of an approved antidepressant were excluded from participation. Using additional psychotropic medications other than the study drug was prohibited. Patients maintained a stable dose of their Parkinson’s disease medication throughout the trial. All evaluations were completed in the “on” state.
Thirteen additional patients signed consent but did not qualify for participation due to failure to meet the inclusion/exclusion criteria described above. Of the 52 patients who were enrolled, 20 met the a priori criteria for entry into the extension phase of the study (i.e., CGI-I of 1, 2, or 3) and chose to continue blinded treatment.
Measures
Cognition was assessed with a battery of neuropsychological tests designed to evaluate the aspects of cognitive functioning that may be affected in Parkinson’s disease. These included the forward and backward digit span subtests of the Wechsler Adult Intelligence Scale—Third Edition (WAIS-III),
27 which assess auditory attention; the word list recall and recognition subtests of the Wechsler Memory Scale-Third Edition (WMS),
28 which measure verbal memory; the Boston Naming Test (BNT)
29 and verbal category fluency test (animal naming), which assess different aspects of language; and the Stroop Color and Word Test,
30 a measure of both processing speed and executive function (i.e., set switching).
Measures of depression (HAM-D, CGI-I), anxiety (Hamilton Anxiety Rating Scale [HAM-A]
31 ), sleep (Pittsburgh Sleep Quality Index [PSQI]
32 ), quality of life (Medical Outcomes Study Short Form [SF-36]
33 and Parkinson’s Disease Questionnaire [PDQ-8]
34 ), and motor functioning (Unified Parkinson’s Disease Rating Scale [UPDRS]
35 ) were also administered over the course of the trial.
Procedure
Preliminary screening was conducted by telephone. Appropriate individuals were scheduled for an in-person evaluation where a detailed medical and psychiatric history was obtained via clinical and semistructured interviews (SCID), a motor exam was performed (UPDRS), and baseline assessments of depression and anxiety were administered (HAM-D, HAM-A). Patients also completed a packet of self-report measures (PSQI, PDQ-8, SF-36) and a battery of neuropsychological tests (MMSE, Digit Span, recall and recognition subtests of the WMS, Stroop, animal naming, and BNT).
Eligible individuals were randomized, in variable length blocks, to receive equivalent-appearing nortriptyline, paroxetine CR, or placebo. Dosing was flexible (based on ranges typical for a geriatric population), and decisions on dose were made at each visit based on efficacy and tolerability, or between visits if the patient was having troublesome side effects (i.e., dry mouth, insomnia). The minimum to maximum doses of study drug were as follows: paroxetine CR, 12.5 mg to 37.5 mg; nortriptyline, 25 mg to 75 mg; placebo, 1–3 pills. All patients were instructed to take a single daily dose of the study medication in the evening. All study personnel were blind to group assignment. Neuropsychological testing and the assessments of depression, anxiety, motor function, sleep, and quality of life were readministered at the end of the acute (week 8) and extension phases of the study (week 24).
RESULTS
Data were analyzed using SPSS version 15 for Windows. All tests were two-tailed. Data analysis included all patients who had a baseline and at least one follow-up neuropsychological assessment. The results presented below detail the impact of successful antidepressant treatment on cognition in Parkinson’s disease and cognitive predictors of treatment response. The impact of antidepressant treatment on mood, quality of life, and motor functioning is detailed elsewhere.
19,
20Fifteen patients (28.8%) were treatment responders (>50% reduction from baseline to week 8 on HAM-D score) while 37 patients (71.2%) were classified as nonresponders. In addition to comparing the impact of treatment response versus nonresponse on cognition, several additional between-group comparisons are reported below. In all of these analyses, the most impaired subgroup or quartile (i.e., highest scores for depression or disease severity; lowest score for memory or executive function) is compared to the rest of the sample. We chose this grouping so that the whole sample could be included in the analyses and because we were most interested in understanding the differences between patients with more severe levels of disease pathology versus those with lower levels of symptomatology.
Baseline Data
Of the 52 patients enrolled in the trial, 48 had a diagnosis of major depression. Two patients were diagnosed with double depression (dysthymia in addition to major depression), while two had only dysthymia. Eighty percent of the cases of major depression were recurrent in nature. The mean age of the sample was 62.2 years old (SD=8.7), the mean duration of Parkinson’s disease was 6.6 years (SD=5.9), the average age of onset was 56 years old (SD=9.5), and the mode of the sample with regard to stage of illness (Hoehn-Yahr scale) was 2. The average dose of medication was 28.4 mgs for paroxetine CR, 48.5 mgs for nortriptyline (with a mean nortriptyline level of 74.88) and 2.7 pills for placebo.
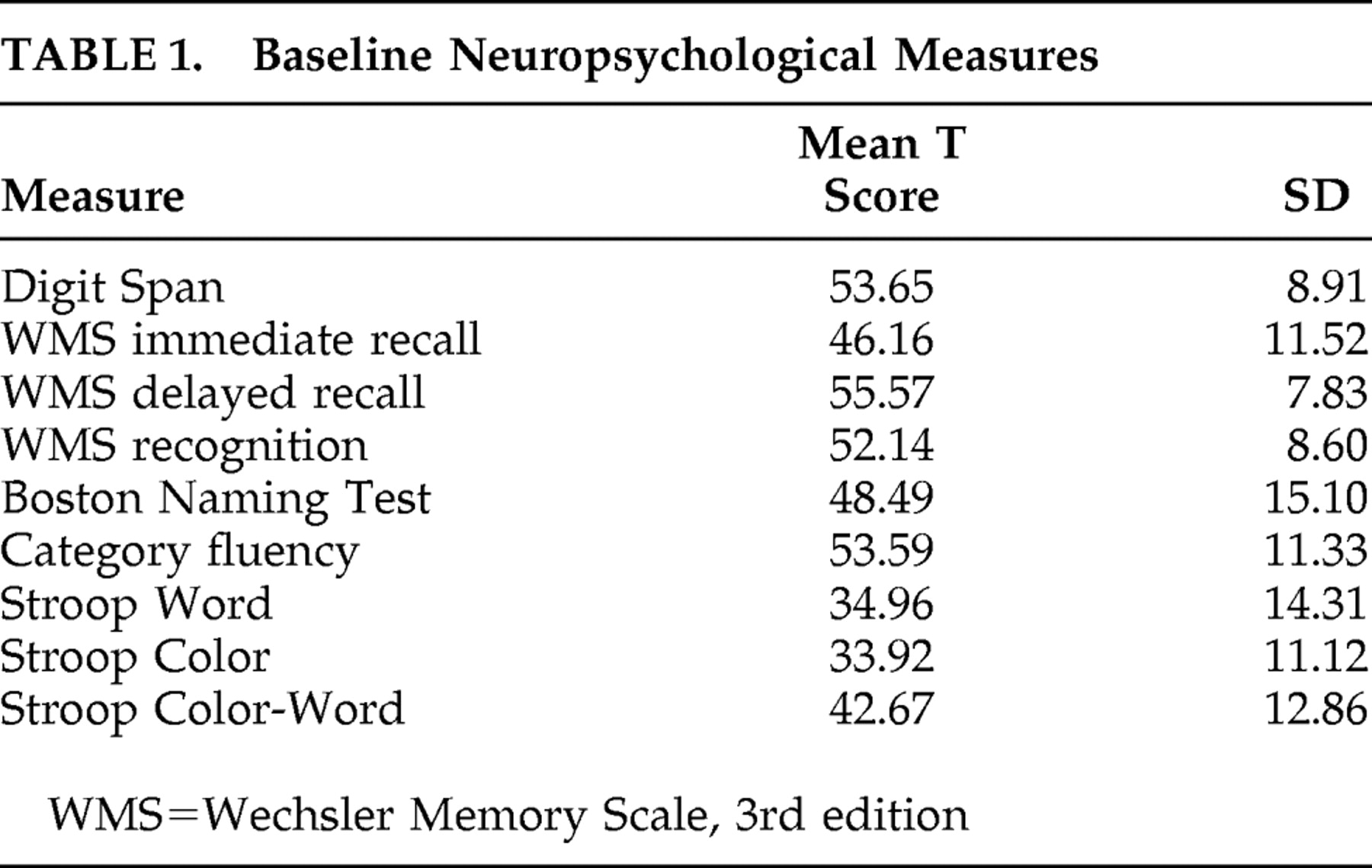
Mean baseline scores on neuropsychological measures of attention, memory, and language all fell within the average range in this sample. The sample as a whole scored well below average on the Word and Color subscales (speed of processing) of the Stroop test. Because patients were so impaired in these areas, no Stroop effect was observed (i.e., mean scores on Color-Word, the executive function portion of the task, were higher than mean scores on the Word and Color subscales). See
Table 1 . In addition, one-way ANOVAs indicated that there were no significant differences between drug groups on any of the baseline neuropsychological measures (p values range from 0.17 to 0.98).
Baseline Cognition, Depression, and Duration of Parkinson’s Disease
Exploratory t tests were conducted to compare the neuropsychological test results of patients who scored in the top quartile for depression (i.e., HAM-D score >22) to those with ratings in the bottom three quartiles (HAM-D score range of 10–21). While there was no significant difference between patients with higher versus lower depression on the MMSE (p=0.89), patients with higher levels of depression performed significantly worse on baseline measures of language (BNT: t=−2.35, df=49, p=0.02; category fluency: t=−3.32, df=49, p=0.002) and memory (immediate recall: t=−1.97, df=49, p=0.05; delayed recall: t=−2.20, df=49, p=0.03) compared to those with less severe depression ratings.
Exploratory t tests also indicated that longer duration (top quartile 10–20 years) versus shorter duration (bottom 3 quartiles 1–9 years) of Parkinson’s disease was associated with poorer performance on both the Word subscale of Stroop (speed of processing: t=−2.87, df=49, p=0.006) and composite Stroop test (speed of processing and executive functions [attention/response inhibition]: t=−2.05, df=50, p=0.05). However, there was no significant difference in baseline depression scores between patients who had Parkinson’s disease for a longer versus shorter period of time (p=0.81). In addition, no significant difference in baseline depression was found between those who scored highest (top quartile) versus lowest (bottom 3 quartiles) on the measures of disease severity (UPDRS total and motor subscale scores, p=0.59 and 0.10, respectively).
Baseline Cognition and Response to Treatment
Exploratory t tests indicated that treatment responders (>50% reduction in baseline to week 8 HAM-D score) had significantly higher baseline scores (i.e., were less impaired) on measures of speed of processing/executive functioning (Stroop composite score: t=2.80, df=50, p=0.007; Stroop Word: t=2.45, df=49, p=0.018; Stroop Color: t=2.53, df=49, p=0.015) and memory (composite score reflecting word list immediate recall, delayed recall, and recognition: t=2.05, df=50, p=0.046; individual memory subscales n.s.).
Acute Phase of Treatment
Neuropsychological Predictors of Acute Treatment Response
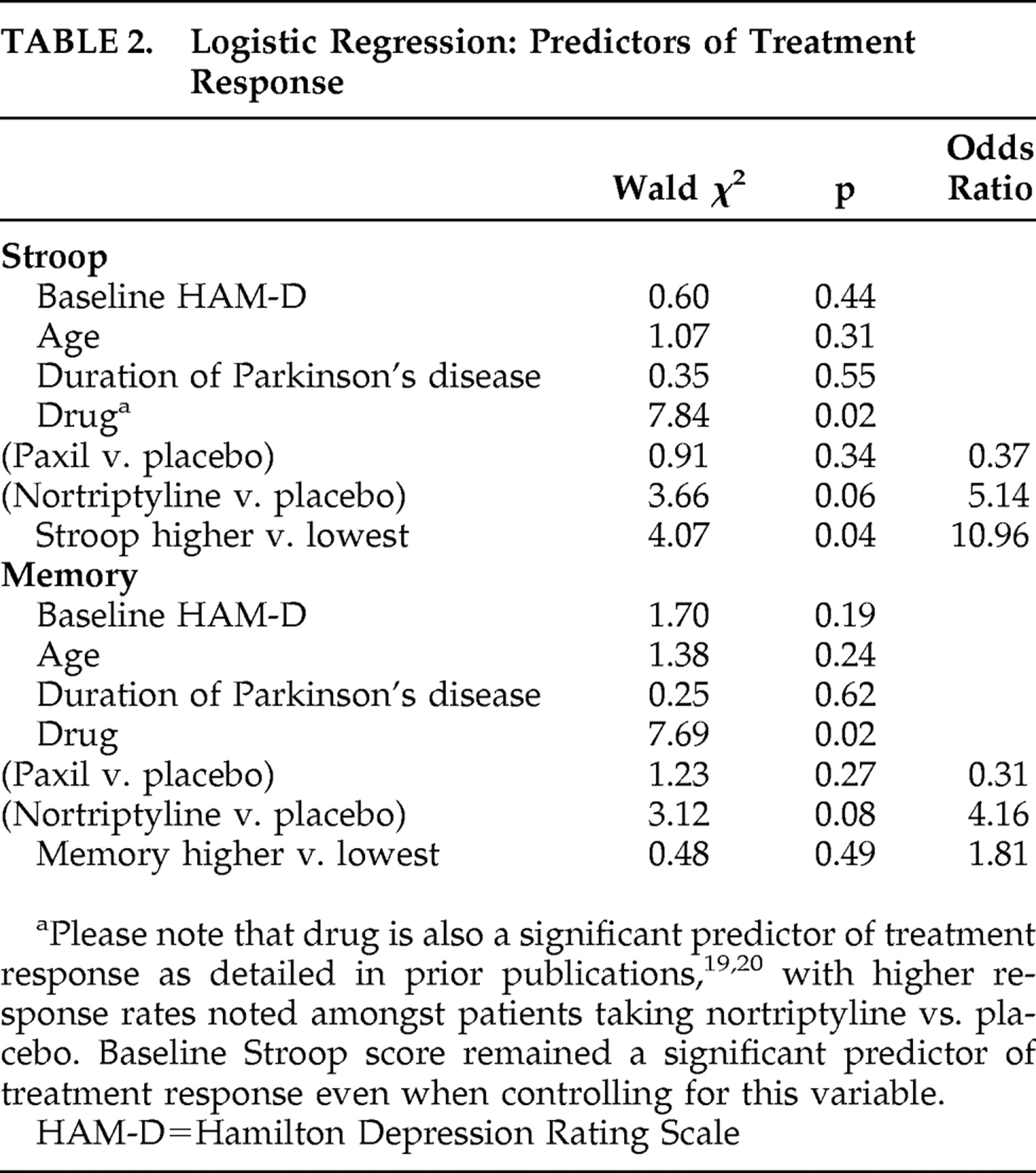
Because patients who were treatment responders in the acute phase had significantly higher baseline scores on measures of both speed of processing/executive functioning and memory, we used logistic regression to examine if higher (top three quartiles; better performance) versus lowest (bottom quartile; poorest performance) scores in these cognitive domains were predictive of treatment response when considering potential confounding variables. When controlling for baseline depression (HAM-D), age, duration of Parkinson’s disease, and the effect of drug (also a significant predictor), a “higher” Stroop composite score at baseline remained a significant predictor of treatment response (Wald χ
2 =4.07, df=1, p=0.04, OR=10.96). A “higher” baseline composite memory score (Wald χ
2 =0.478, df=1, p=0.49, OR=1.81), however, was not a significant predictor of treatment response when controlling for the aforementioned variables (see
Table 2 ).
Effect of Depression Treatment Response on Cognition
Repeated measures ANOVA indicated that there were no significant group (responder status; >50% reduction in baseline to week 8 HAM-D score) by time interactions on any of the neuropsychological measures in the acute treatment phase (p values from 0.10 to 0.889). Therefore, depression “responders” did not demonstrate larger improvements in cognition than nonresponders. In addition, there was no correlation between change in patients’ HAM-D scores and change in their performance on any neuropsychological measure between baseline and week 8 (p values from 0.55 to 0.78).
Extension Phase of Treatment
Because only 20 patients entered the extension phase of the study, there was not sufficient power to examine neuropsychological predictors of long-term treatment response or to compare differences between responders and nonresponders in this phase of treatment. However, we did examine the impact of longer-term treatment of depression on cognition for all patients who met the criteria to enter the extension phase of the study (at least minimally improved on the CGI-I after 8 weeks of treatment). Results of repeated measures ANOVA suggested that patients who entered the extension phase of the study demonstrated significant improvements in verbal memory (composite score: F=7.93, df=2, 17, p=0.004; immediate word recall: F=9.12, df=2, 16, p=0.002; word recognition: F=5.50, df=2, 16, p=0.02; word delayed recall: F=6.09, df=2, 16, p=0.01) and one test of language (BNT: F=6.37, df=2, 16, p=0.009) over the course of the study. Planned contrasts indicated that significant improvements in the verbal memory composite score (F=16.70, df=1, 18, p=0.001) and immediate recall (F=16.97, df=1, 17, p=0.001) were evident by the end of the acute phase and maintained throughout the end of the extension phase (composite: F=7.72, df=1, 18, p=0.01; immediate recall: F=6.26, df=1, 17, p=0.023). Gains specific to delayed recall (F=12.36, df=1, 17, p=0.003) and recognition (F=11.40, df=1, 17, p=0.004) were observed at week 8, but week 24 scores on these domains were not significantly different from baseline (0.10 and 0.15, respectively). Improvements on the Boston Naming Test, however, were not apparent until the extension phase of the study (F=13.05, df=1, 17, p=0.002) (i.e., no change between baseline and week 8, but significant change noted between baseline and week 24). No notable changes were observed in a second test of language (verbal fluency-animal naming, p=0.257) or measures of attention (digit span: p=0.386) or executive function (Stroop: p values from 0.258 to 0.692).
Effect of Drug on Cognition: Acute and Extension Phase
Repeated measures ANOVA indicated that there were no significant group (drug) by time interactions on any of the neuropsychological measures in either the acute (p values from 0.10 to 0.89) or follow-up period (p values from 0.15 to 0.90). Therefore, neither paroxetine, nortriptyline, nor placebo was associated with either an improvement or worsening of cognitive functioning in either short- or longer-term treatment.
DISCUSSION
This is one of few studies to examine the impact of antidepressant treatment on cognition in patients with Parkinson’s disease and depression. Overall, results indicated that higher baseline scores on measures of speed of processing and executive functions (Stroop) predicted acute treatment response, even when controlling for confounding factors such as age, duration of Parkinson’s disease, baseline depression, and drug effect (with nortriptyline superior to placebo for the acute treatment of depression as detailed elsewhere
19,
20 ). However, while these aspects of cognitive functioning appeared to predict short-term treatment response in this population, no area of cognition was found to improve as a result of successful antidepressant treatment after the end of the 8-week acute phase. Patients who demonstrated “response” to antidepressant treatment scored higher on baseline measures of cognition (i.e., verbal memory, speed of processing, executive functioning), compared to patients who did not respond, but their scores did not improve over the course of treatment. Moreover, neither paroxetine nor nortriptyline appeared to have negative effects on cognition in the context of short (8 weeks) and longer-term (24 weeks) antidepressant treatment. Finally, consistent with past cross-sectional studies,
8 –
12 more severe depression was associated with poorer performance on baseline tests of memory and language, and no Stroop effect was observed.
36,
37There are few studies with which to compare these results in Parkinson’s disease. In one of the limited studies conducted within the Parkinson’s disease with depression population, Weintraub et al.
17 also found higher baseline scores of verbal memory to be associated with increased rates of treatment response with escitalopram, and that treatment “response” was not associated with any type of improvement across a variety of cognitive domains.
17 However, in contrast to our study, these authors did not find an effect for either baseline psychomotor speed or executive functions on treatment outcome. Yet the small sample size and limited rates of response in Weintraub et al.’s
17 study make it difficult to aggregate these findings. In addition, dopamine agonists, such as pramipexole, have been shown to have negligible effects on working memory and attention in Parkinson’s disease depression, despite their potential antidepressant effects.
18Several of our findings are also consistent with the geriatric depression literature. For example, speed of processing, verbal memory, and executive functions have been found to be predictive of antidepressant treatment response in several studies in the aged.
14 –
16 Moreover, while research concerning change in cognitive status is mixed, some studies have indicated that efficacious antidepressant treatment is not associated with cognitive gains in the elderly.
38 –
40 Furthermore, one study found that older patients with little cognitive impairment prior to treatment did not experience cognitive gains following antidepressant therapy whereas patients who were more impaired did exhibit improvements following treatment.
40Therefore, one possibility for our finding—that responders did not improve more than nonresponders on neuropsychological measures after acute treatment—may be the fact that patients did not demonstrate gross impairments on the neuropsychological measures at baseline (with the exception of Stroop). This pattern of average baseline performance and lack of change over time is consistent with that observed by Rektorová et al.
18 in their study investigating the efficacy of dopamine agonists on depression and cognition in Parkinson’s disease. It is also likely that the small sample size, the limited range of scores observed on many of the neuropsychological measures (potentially because the majority of our patients presented in the earlier stages of Parkinson’s disease), and the mild to moderate levels of depression reported by most of the sample (as more severe depression has a greater deleterious impact on cognition
41 ) restricted our ability to detect cognitive changes between responders and nonresponders.
Alternatively, it is possible that the neuroanatomical changes that characterize Parkinson’s disease (i.e., degeneration of dopaminergic cells in the substantia nigra, dysfunction of cortico-striatal circuits instrumental in frontal brain functions, and presence of diffuse Lewy bodies
13 ) are the main contributor to the cognitive deficits observed in this population. For example, because Stroop score (predictive of treatment response) was associated with Parkinson’s disease duration but not depression at baseline, poor Stroop performance may be more sensitive to the effects of Parkinson’s disease than those of depression.
42 As a result, poor performance on this test may index more severe disease and widespread neuropathology, making treatment response less likely for this reason. For the same reason, and because poor performance on select cognitive tasks (such as Stroop) may not be related to depression, successful treatment of depression in Parkinson’s disease may exert minimal impact on certain aspects of cognition (i.e., executive functions, speed of processing) as it cannot reverse the structural brain changes inherent in the disease process.
Limited conclusions may be drawn from the extension phase data given the small sample size and lack of power needed to make between-group comparisons. Yet it is interesting to note that improvements in language (i.e., naming) were not observed until the extension phase of the trial for patients who opted to continue with blinded treatment. This finding may suggest that longer term treatment of depression may lead to sustained improvement in this cognitive domain. However, in the absence of a comparison condition (all patients who entered the extension phase were at least partially improved, though not necessarily “responders”), interpretation of this finding is difficult, and the role of practice effects, though thought to be small in Parkinson’s disease,
43 cannot be dismissed.
In conclusion, our findings suggest that higher baseline performance on measures of executive functioning, speed of processing, and verbal memory was associated with antidepressant treatment response in Parkinson’s disease. However, “responder status” was not linked with any improvements or changes in cognitive status during the acute phase of treatment. Improvements in language noted during the extension phase must be interpreted with caution given the absence of a comparison condition. As this is one of few studies examining the impact of treatment of depression on cognition in Parkinson’s disease, further research is needed to replicate these findings.
Acknowledgments
This study was supported by a grant from the National Institute of Neurological Disorders and Stroke (NINDS) RO1NS043144. GlaxoSmithKline provided free paroxetine CR and matching placebo. Clinical Trials Registration: Clinicaltrials.gov Identifier: NCT 00062738.
Dr. Dobkin has received research support from NIH (NINDS). Dr. Menza has received research support from NIH (NINDS), Astra-Zeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Forest Laboratories, GlaxoSmithKline, Lilly, Pfizer, Sanofi-Aventis, Sepracor, Takeda Wyeth; has served as a consultant for NIH (NIMH, NINDS), GlaxoSmithKline, Kyowa, Lilly Research Laboratories, Pfizer, Sepracor, Takeda Wyeth; and was a speaker for Sanofi-Aventis. Dr. Marin has received research support from NIH (NINDS), GlaxoSmithKline, Lilly, Sanofi-Aventis, Sepracor, and Takeda Wyeth, and served as a consultant for Lilly Research Laboratories. Dr. Mark has received research support from Kyowa and Cephalon and served as a speaker for Allergan, Boehringer Ingelheim, GlaxoSmithKline, and Valeant. Dr. Tröster has received research support from Medtronic and served as a consultant for Medtronic and Advanced Neuromodulation Systems.