D espite the introduction of highly active antiretroviral treatment (HAART), individuals infected with HIV frequently exhibit neuropsychiatric symptoms including the more severe HIV-associated dementia; a less symptomatic, but significantly impairing, mild neurocognitive disorder
1,
2 ; and apathy. Apathy or decreased motivation in HIV can affect performance on nearly all aspects of cognitive, instrumental, and interpersonal functions.
3 It is unclear whether apathy in HIV patients reflects a direct effect of the virus on subcortical brain regions or a secondary neuropsychiatric symptom. We investigate whether apathy in the absence of cognitive decline and depression results from damage to white matter using diffusion tensor imaging (DTI).
While HAART is effective in many who will develop neurocognitive disorders in HIV,
4 evidence suggests that many will develop these disorders earlier than current treatment regimes would sanction initiation of treatment.
5 It is also not known in the earlier phases of the disease who will go on to develop neurocognitive disorders, nor who will respond to treatment.
6 The neurotoxic effects of HIV result in damage to white matter tracts in the brain.
7 Once damage is established and related cognitive disorders ensue, the ability of HAART to reverse existing dysfunction is probably limited.
8 Earlier treatment with HAART in at-risk or minimally symptomatic patients may prevent further decline in cognition and delay the course of HIV disease. Identification of reliable early behavioral and imaging correlates of risk for subsequent cognitive dysfunction, such as apathy, become all the more crucial to delineate.
Apathy refers to reduced self-initiated cognitive, emotional, and behavioral activity.
9 Apathy has been recognized as a potential early behavioral feature of HIV-associated dementia.
10,
11 It occurs along with other symptoms of subcortical dysfunction including memory impairment, poor concentration, and reduced psychomotor speed.
12 Apathy has also been reported to be prevalent in HIV patients without dementia,
13 and as such may not be confined to late-stage disease (i.e., people with CD4 cell counts <300).
14 Depression, often associated with apathy, is distinct in that it is fundamentally a disturbance of mood, and it is frequently characterized by limitations to the capacity to enjoy previously pleasurable activities (anhedonia). These two syndromes have been observed both alone and concurrently in a variety of neurological and psychiatric disorders, including Parkinson’s disease
15 and Alzheimer’s disease.
16 Both depression and apathy have been associated with higher risk of cognitive decline in the later stages of HIV, which in turn predicts more rapid progression to AIDS.
1,
2 Depression has its neuroanatomical basis in the left prefrontal cortex and limbic system, while the emergence of apathy is thought to be based on medial prefrontal and deep subcortical areas, such as the nucleus accumbens.
To date, there has been relatively limited investigation of apathy and HIV-associated neurocognitive disorders (HANDs) in HIV. Structural MRI studies have found an inverse correlation between apathy levels and lower nucleus accumbens volumes. Ratings of depression were unrelated to either apathy or nucleus accumbens volume.
10 Although Paul et al.
10 suggested that apathy may represent a behavioral phenotype of HIV brain involvement, the presence of confounding variables such as coexisting substance abuse and previous head injuries limits the interpretation of these findings. Also, due to the lack of a nonapathetic HIV+ comparison group, this study was unable to address the question of the primary role of HIV in the gray matter volume reduction seen in the nucleus accumbens of apathetic HIV+ people.
Diffusion weighted imaging used to derive measures of fractional anisotropy in white matter tracts provides an indirect indication of the structural integrity of the regional white matter.
17 Previous work in HIV has shown that changes in brain function occur in a presymptomatic phase of cognitive illnesses in the presence of normal structural MR studies.
18 Previous studies using DTI, arguably a more sensitive measure of white matter integrity than conventional structural MR sequences, have demonstrated frontal and internal capsular pallor in clinical studies of HIV-1 positive patients.
19 Others have reported a trend toward reduced fractional anisotropy in the frontal white matter, the corpus callosum, and hippocampus of HIV+ individuals relative to comparison subjects,
20 or that diffusion abnormalities in the splenium of the corpus callosum were associated with dementia severity and motor speed losses.
21 As such, preliminary evidence suggests that there is white matter involvement in HANDs, but as yet, no study of which we are aware has investigated the correlation with changes in white matter and a putative behavioral marker of subsequent cognitive decline such as apathy.
Consequently, the present study, which sought to extend the work by Paul et al.,
10 suggests that apathy may be a useful behavioral marker of dementia by examining correlations of white matter brain changes with apathy in HIV in the absence of depression or other psychiatric comorbidity including cognitive impairment. We hypothesized that apathy would be specifically associated with white matter changes reflected as lower fractional anisotropy using DTI in the medial prefrontal brain region of the white matter regions linking the anterior cingulate, the nucleus accumbens, and other frontal cortical regions.
METHODS
Subjects
Participants (n=30) with HIV Stage III disease and healthy HIV− comparison subjects (n=10), ages 18–45 years old, were recruited from the Infectious Diseases Clinic at Tygerberg Hospital and the surrounding community in greater Cape Town. To be eligible, HIV patients had to have been on HAART for a minimum of 3 months. All participants had to be cognitively normal on screening tests (International HIV Dementia Scale score >10) and be free of psychotropic medication and have had no recent (within 6 months) substance abuse history. The presence of other current DSM-IV disorders, determined through semistructured interviews using the Mini-International Neuropsychiatric Interview,
22 was also an exclusion criterion. HIV diagnosis was based on serologic testing by ELISA and Western blot. Patients were excluded if they reported a history of head injury with loss of consciousness exceeding 10 minutes.
Procedure
Participants completed clinical assessments in a single testing session. Neuroimaging was conducted within 1 week of the initial evaluation. All measures were administered and scored according to standard procedures. Written informed consent was obtained prior to enrollment and exposure to any study-related procedure. The protocol was approved in writing by the Committee for Human Research of the University of Stellenbosch.
Apathy
Apathy was measured using the clinician-rated Apathy Evaluation Scale. The Apathy Evaluation Scale is comprised of 18 items that relate to motivation, self-initiation, and drive and has sound psychometric properties.
23 Participants are asked to register their degree of agreement with each question using a four-point Likert scale. Higher scores reflect greater apathy (1=not at all, 4=extremely). Excluding significant mood symptoms even though subjects did not meet criteria for a major depressive disorder was important to ensure careful distinction of potentially overlapping symptoms of depression and apathy. We used the 14-item clinician-rated Hamilton Depression Rating Scale (HAM-D)
24 which has been shown to reliably differentiate depressed from nondepressed subjects with HIV.
25 It has also been widely used in treatment studies of depression in HIV. A HAM-D score of 16 (moderate depression) was taken as a cutoff for significant depressive symptoms and served as an additional exclusion from the cohorts.
To screen for the presence of HANDs, we used the well-validated International HIV Dementia Scale,
26 a brief and reliable measure of global cognitive function that is reliably able to identify features of HIV-associated dementia. A neuropsychological test battery was administered to all participants to assess specific domains of attention, concentration, learning, memory, psychomotor speed, and executive function. We used the major components of the MATRICS Consensus Cognition Battery.
27,
28 Speed of processing was assessed with the symbol coding test (digit-symbol matching) and the Trail Making test, part A.
29 Attention and vigilance were assessed by a computerized version of the continuous performance test. We examined working memory using the Weschler Memory Scale for Nonverbal Memory (spatial span test) and the letter/number span test for verbal memory.
29 For verbal learning, the Hopkins Verbal Learning Test—Revised was used,
30 and animal naming for category/verbal fluency. Visual learning and visuospacial function were examined using the Visual Special Memory Test (revised edition). For reasoning and problem solving (planning and reasoning ability), we used neuropsychology assessment battery (NAB) mazes.
A full neuromedical examination was conducted on all participants. Blood for CD4 cell counts and HIV RNA load (20 ml) were taken in the infectious diseases clinic. Urine for screening of illicit drug use was taken at screening.
Brain Imaging
Imaging was performed at The Cape Universities Brain Imaging Centre (CUBIC) on a Siemens Magnetom 3T Allegra within 7 days of the screening and neuropsychological assessment. We acquired a multidirectional diffusion weighted sequence with 30 diffusion directions, 1 b=0 sec/mm 2 direction, TR=8,800 msec, TE=88 msec, and a b-value=1,000 sec/mm 2 . The images were acquired as a mosaic resulting in a 960×960 matrix with 60 slices per volume and a corresponding in-plane spatial resolution and slice thickness of 2×2 mm 2 and 2.2 mm, respectively. The 2.30 minute sequence was repeated three times and averages were derived.
Image Processing
Preprocessing of the images was conducted in FMRIB Software Library and MATLAB respectively. First the images underwent eddy current correction in FSL. The tensor residuals for each pixel in the images were then computed and any outliers were removed, resulting in an average DTI and fractional anisotropy map for each subject. An affine as well as a nonlinear registration of the B0 images to each subject’s structural T1 image (intra-subject) was performed. The warps were applied to the fractional anisotropy images. A mean fractional anisotropy template was then created from all the subjects, followed by a final registration and warp of all the fractional anisotropy images to the mean template. A general linear model ANOVA was performed on the fractional anisotropy voxels of each cohort to determine significant differences between the groups. This was performed at a p<0.05 uncorrected threshold with a fractional anisotropy mask of 0.25.
Contrasts between HIV+ groups were then drawn to examine the associations between apathy, performance on neuropsychological tests, and white matter fractional anisotropy. As apathy was not normally distributed among either patient group, nonparametric statistics were conducted for all analyses in which apathy was included.
RESULTS
Of the 40 subjects originally enrolled, 36 participants (HIV+ with apathy, n=13; HIV+ without apathy, n=13; healthy comparison subjects, n=10) were included in the final analysis. Two imaging sequences in each of the HIV+ groups were discarded due to movement artifacts (n=3) and poor quality acquisition (n=1). The mean ages of the apathetic and nonapathetic cohorts were 33.08 years old (SD=3.9) and 34.23 years old (SD=4.3), respectively, which is similar to the average age of presentation with Stage III HIV disease in our adult infectious diseases population. HIV+ participants had been on antiretroviral drugs for a minimum of 3 months. Together, low viral loads and being clinically asymptomatic suggest that HAART is largely effective in this cohort (
Table 1 ). At 22.6 years old (SD=5.2), the average age of the HIV− comparison cohort was younger than either of the HIV+ cohorts, but they were similar with respect to years of education (mean=10 years).
Apathy Evaluation Scale scores in the HIV+ apathetic group (46.00, SD=6.0) were higher than those of HIV+ nonapathetic participants (30.46, SD=5.9) and healthy comparison subjects (24.2, SD=4.5). Depression scores were similar across groups (
Table 1 ).
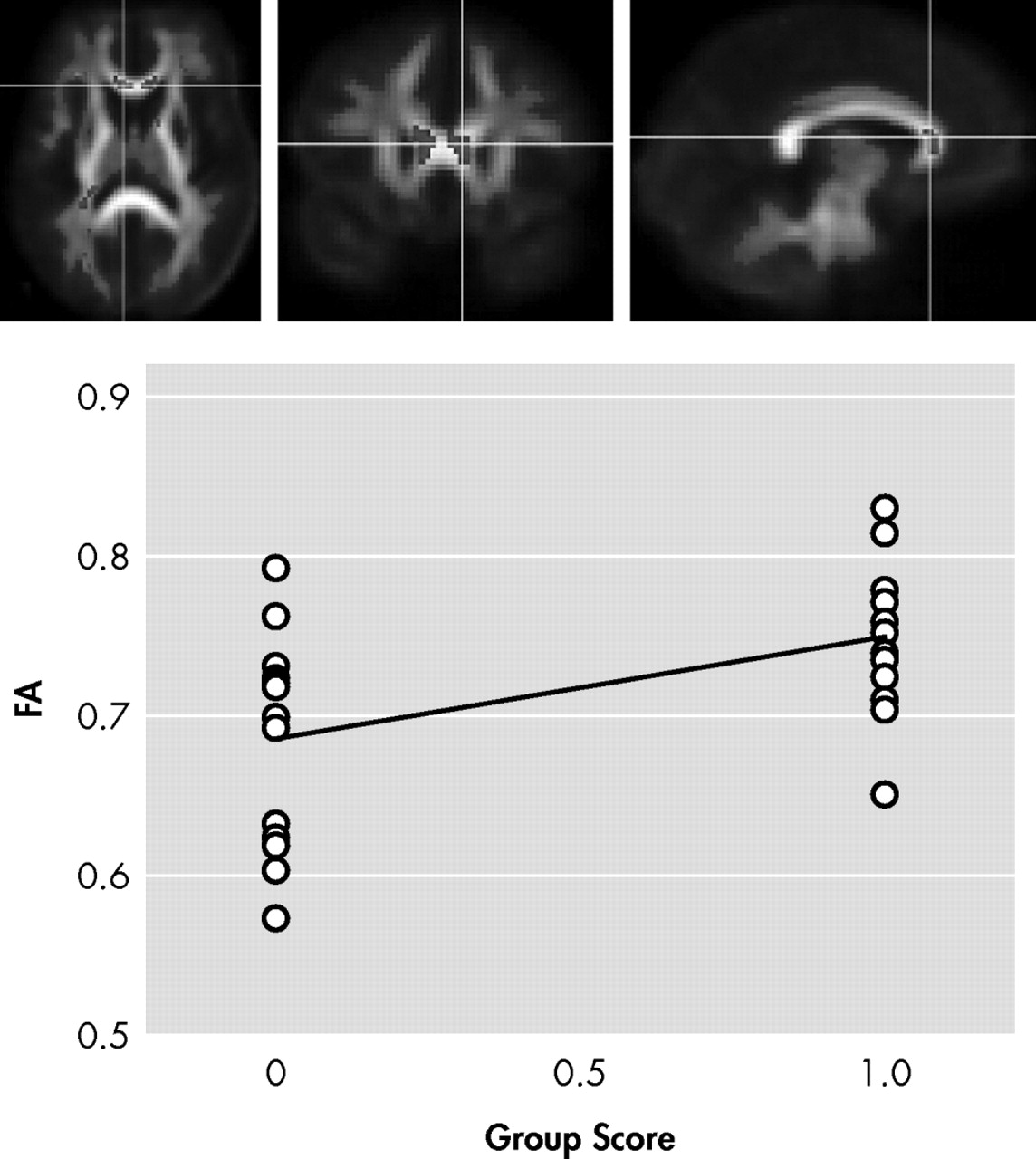
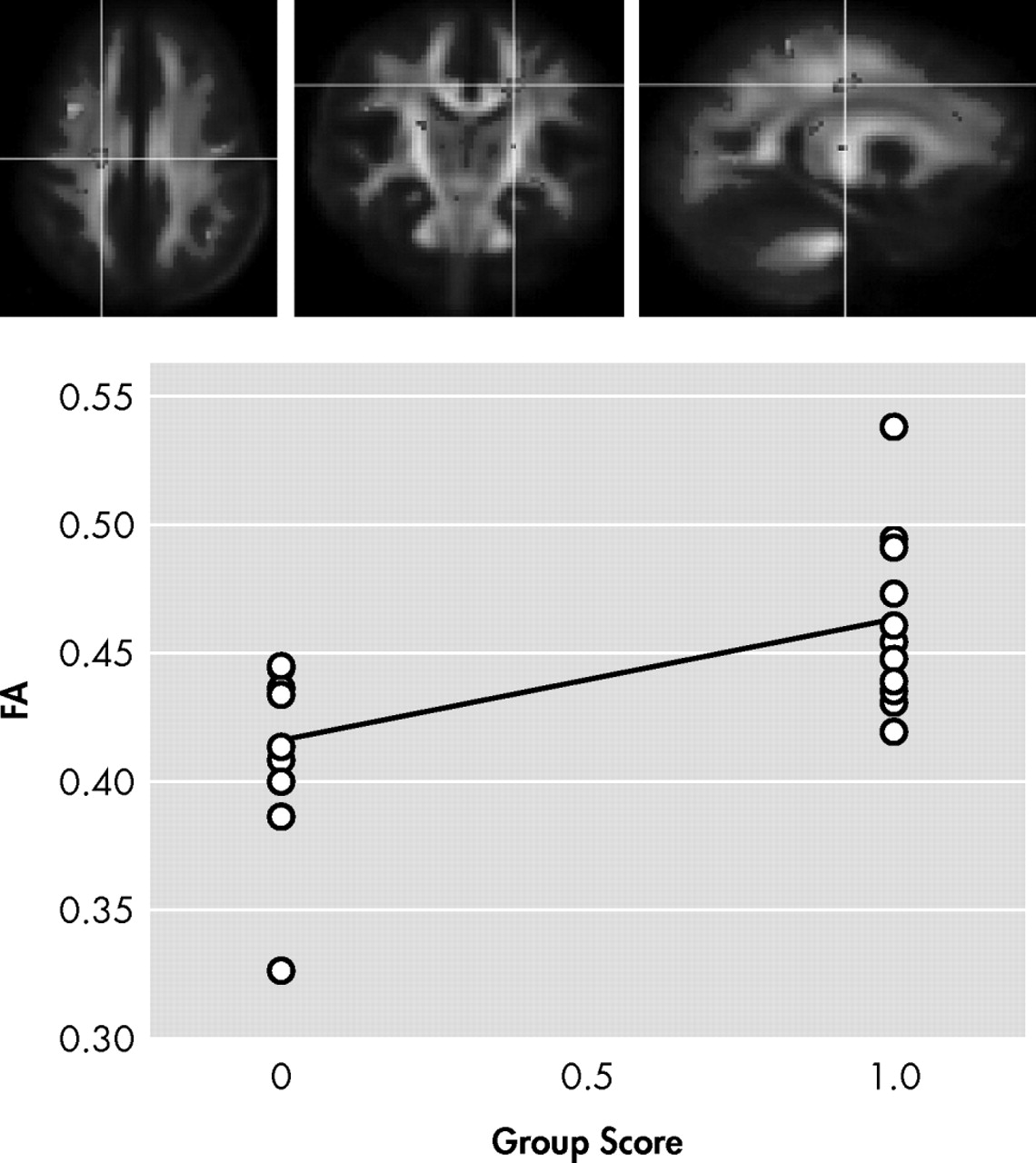
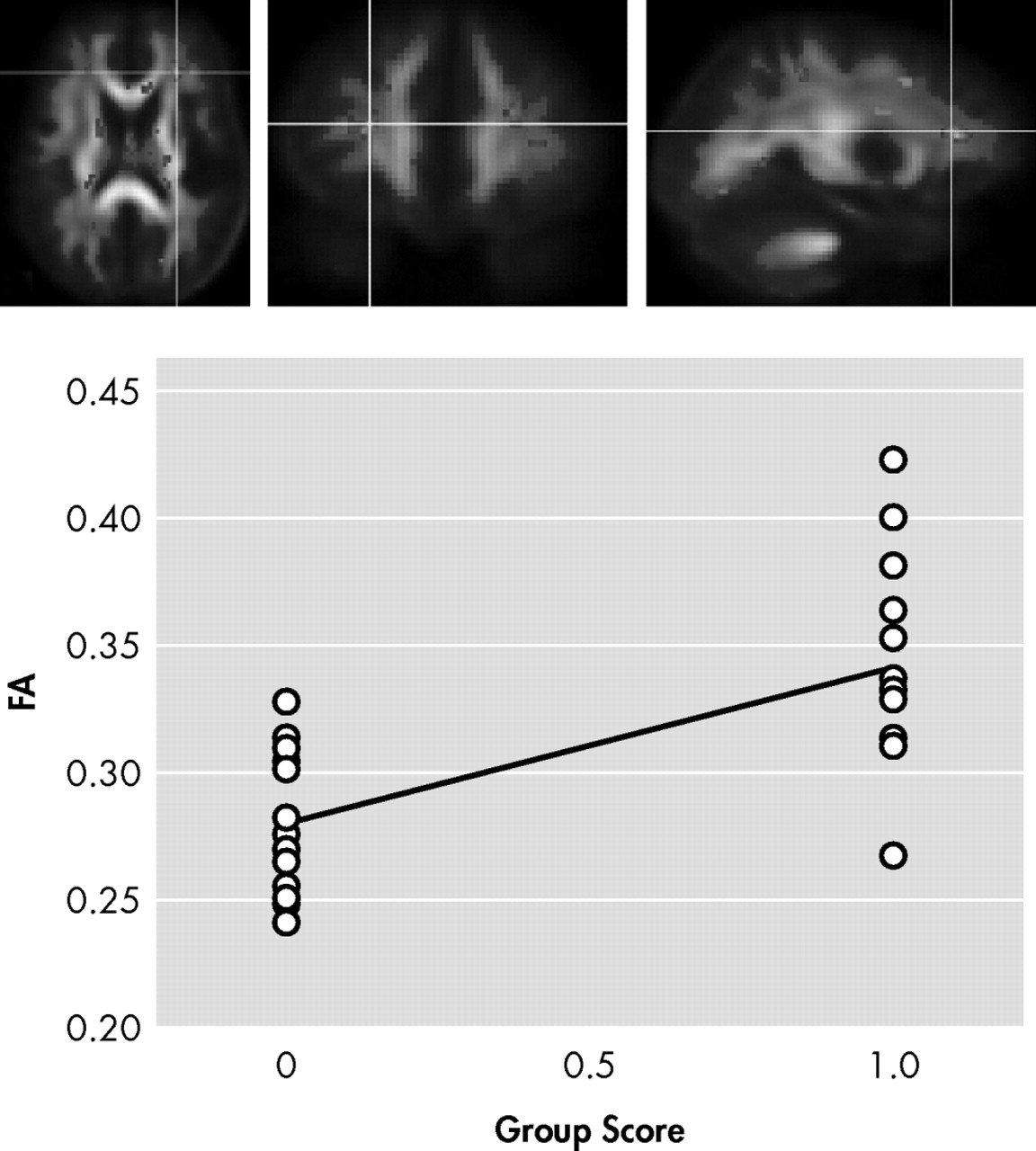
In the first contrast of HIV+ nonapathetic participants versus HIV+ patients with apathy, HIV+ nonapathetic participants demonstrated significantly higher fractional anisotropy values in the genu of the corpus callosum (p=0.002), the superior corona radiata (p=0.0009), and anterior corona radiata (p=0.0002), and the anterior thalamic radiation (p=0.0002) (
Table 3 ;
Figure 1,
Figure 2, and
Figure 3 ).
We also examined the contrasts of healthy comparison subjects versus HIV+ nonapathetic participants. The healthy comparison subjects had significantly higher fractional anisotropy values in the corticospinal tract (p=0.016), the body of the corpus callosum (p=0.039), the anterior thalamic radiation (p=0.011), the superior longitudinal fasciculus (p=0.00017), the superior corona radiata (p=0.012), and the genu of the corpus callosum (p=0.0024) (
Table 3 ).
Regression analyses with fractional anisotropy values as the dependent variable and individual neuropsychological tests scores as independent variables found a significant effect of the Hopkins Verbal Learning Test and mean fractional anisotropy in the superior corona radiata (R=0.613, p=0,039). No other brain region fractional anisotropy was associated with neuropsychological function in any of the cognitive domains tested.
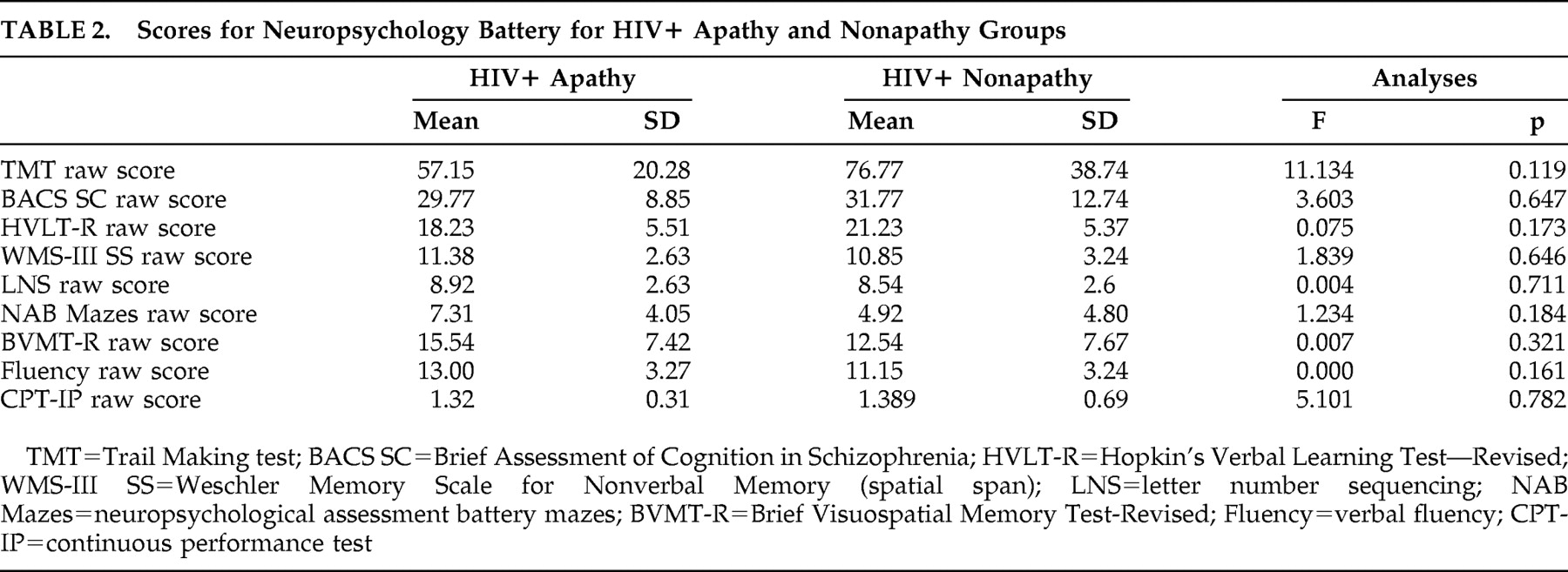
Apathy scores and fractional anisotropy values were negatively correlated in the sagittal stratum, posterior corona radiata, genu of the corpus callosum, superior corona radiata, corticopontine/corticospinal tract, and corticopontine/anteriorthalamic radiation (
Table 2 ) .
Shown in
Figure 1,
Figure 2, and
Figure 3 is the ANOVA voxel-based analysis of the DTI images. The images are shown in the axial, sagittal, and coronal fields, respectively, with the R indicating the right hemisphere. The region of interest is indicated with the crosshairs, and the graph in the lower right corner shows the different subjects in relation to one another. The HIV+ nonapathy group is on the right side of the graph and the HIV+ apathy group is on the left. Each circle represents one subject with n=13 for both groups. The p value shown on the graph is only for the specific point in the cluster; included is the centroid p value for each cluster as well.
HIV+ Nonapathy (n=13) Versus HIV+ Apathy (n=13)
Figure 1,
Figure 2, and
Figure 3 show the decreases in fractional anisotropy (gray) for the HIV+ apathy cohort when compared to the HIV+ nonapathy cohort.
DISCUSSION
The main findings in our study were first that apathy as measured by the Apathy Evaluation Scale was present in a cohort of HIV+ patients in the absence of depression and cognitive impairment. A second DTI revealed lower fractional anisotropy in both the HIV+ groups in the corpus callosum. These were significantly lower in the apathy group, giving a strong indication that this medial brain region is differentially affected by apathy in HIV disease. This suggests that white matter damage is consequent to HIV-mediated neurotoxicity and that in some patients, this damage is clinically expressed as apathy in the absence of a wider or at least symptomatic cognitive disorder. Higher scores of apathy correlated significantly with lower fractional anisotropy values when contrasted with subjects who were HIV+ but were not apathetic.
Consistent with recent studies,
13 we found elevated rates of apathy in patients without dementia and depression. Tate et al.
14 reported that apathy is present even among patients without severe immune suppression. Although symptoms of apathy also overlap with features of depression, the two conditions are unique and have been reliably differentiated in patient samples.
9 HIV has an affinity for
31 the basal ganglia which are critical nuclei in parallel frontal subcortical circuits. These circuits regulate cognition, emotions, and behavioral activation.
32 It is therefore not surprising that apathy and depression are both observed, concurrently and independently, in a substantial percentage of HIV-infected patients.
33Our contrasts of healthy comparison subjects versus HIV+ nonapathetic participants showed HIV-mediated damage to white matter. Healthy comparison subjects had significantly higher fractional anisotropy values in the corpus callosum, the anterior thalamic radiation, the superior longitudinal fasciculus, and the superior corona radiata. Diffusion tensor abnormalities involving the frontal white matter and the corpus callosum have been observed in patients infected with HIV.
19 These include findings of callosal white matter thinning in cognitively asymptomatic patients infected with HIV.
31 The corpus callosum is central to interhemispheric relay and integration, as well as providing a link with subcortical structures, such as the basal ganglia.
34 Injury may be reflected in slowed response initiation and longer reaction times on tasks involving hemispheric transfer or integration between regions.
34 Studies of other CNS disorders characterized by cognitive decline have found evidence that DTI measurements acquired in the corpus callosum may be sensitive to subtle or early changes not apparent on conventional images.
35 This is likely attributable to the lower fractional anisotropy measurement error in regions, such as the corpus callosum, which have intrinsically high anisotropy.
36Results of the present study contrasting HIV+ nonapathetic participants with HIV+ patients with apathy confirm our hypothesis in which we anticipated changes associated with apathy in white matter tracts that relay through the medial prefrontal cortex including the corpus callosum, the anterior thalamic radiation, and the corona radiata. These findings provide evidence that the behavioral phenotype of apathy is associated with neural changes induced by HIV. These results contrast with those of voxel-based morphometry in which we examined regional tissue type differences in volume across the brain and found no statistical differences in brain volumes between HIV+ groups (Moodliar et al., unpublished data, April 2009). Our findings here are consistent with current neuroanatomical models which emphasize the role of medial and dorsolateral prefrontal cortex including the anterior cingulate cortex in the initiation and execution of complex tasks,
37 for behavioral drive and dysfunction being seen as the apathetic behavioral syndrome.
38,
39Limitations of our study include the absence of full neuropsychological assessments in our healthy comparison subjects. We do, however, have data on years of education, making it reasonable to assume that they had normal neuropsychological function overall. Our sample size is small, and our data are cross-sectional and as such are not able to address the question of whether apathy represents an early marker of subsequent cognitive decline.
Despite these limitations, findings here revealed lower fractional anisotropy in both HIV+ groups in the corpus callosum; these were significantly lower in the apathetic group, giving a strong indication that this medial brain region is differentially affected by apathy in HIV disease. Future work could usefully focus on anisotropy measurements of the corpus callosum as a quantitative imaging biomarker in the setting of HANDs using larger longitudinal studies. Studies examining response to HAART treatment in patients infected with HIV will be important to determine whether DTI abnormalities observed in the corpus callosum and other regions reflect reversible or more advanced irreversible injury. Additional work is needed to examine the clinical and functional relevance of white matter disconnectivity and neuropsychiatric symptoms such as apathy in HIV in greater detail. Correlates of white matter damage and neurocognitive decline need to be sought, including whether measures of central viral load correlate with poor fractional anisotropy values, illness duration, age, treatment exposure and treatment adherence. These factors are almost certainly critical in determining the overall impact of HIV on brain function, and in particular on white matter integrity.
Acknowledgments
Partial funding for this study was received from the Harry Crossley Foundation. This work was presented at the South African Society of Psychiatrists, Biological Psychiatry Congress, Kleinmond, South Africa, on May 28–31, 2009. The work was conducted at Cape Universities Brain Imaging Centre, Faculty of Health Sciences, University of Stellenbosch, Tygerberg, 7505.