T raumatic brain injury (TBI) is a major health concern,
1 and long-term TBI survivors display significant neurological and psychiatric impairments.
2 TBI is a heterogeneous condition featuring spatially and temporally disseminated necrotic and apoptotic cell death.
3 Apoptosis,
4 excitotoxicity, and mitochondrial impairment play a decisive role,
5 and the time scale of these changes has been reported to span several months. Delayed neuronal death occurs in vulnerable sites such as the hippocampus,
6,
7 whose damage is considered a key factor responsible for cognitive and affective alterations secondary to TBI.
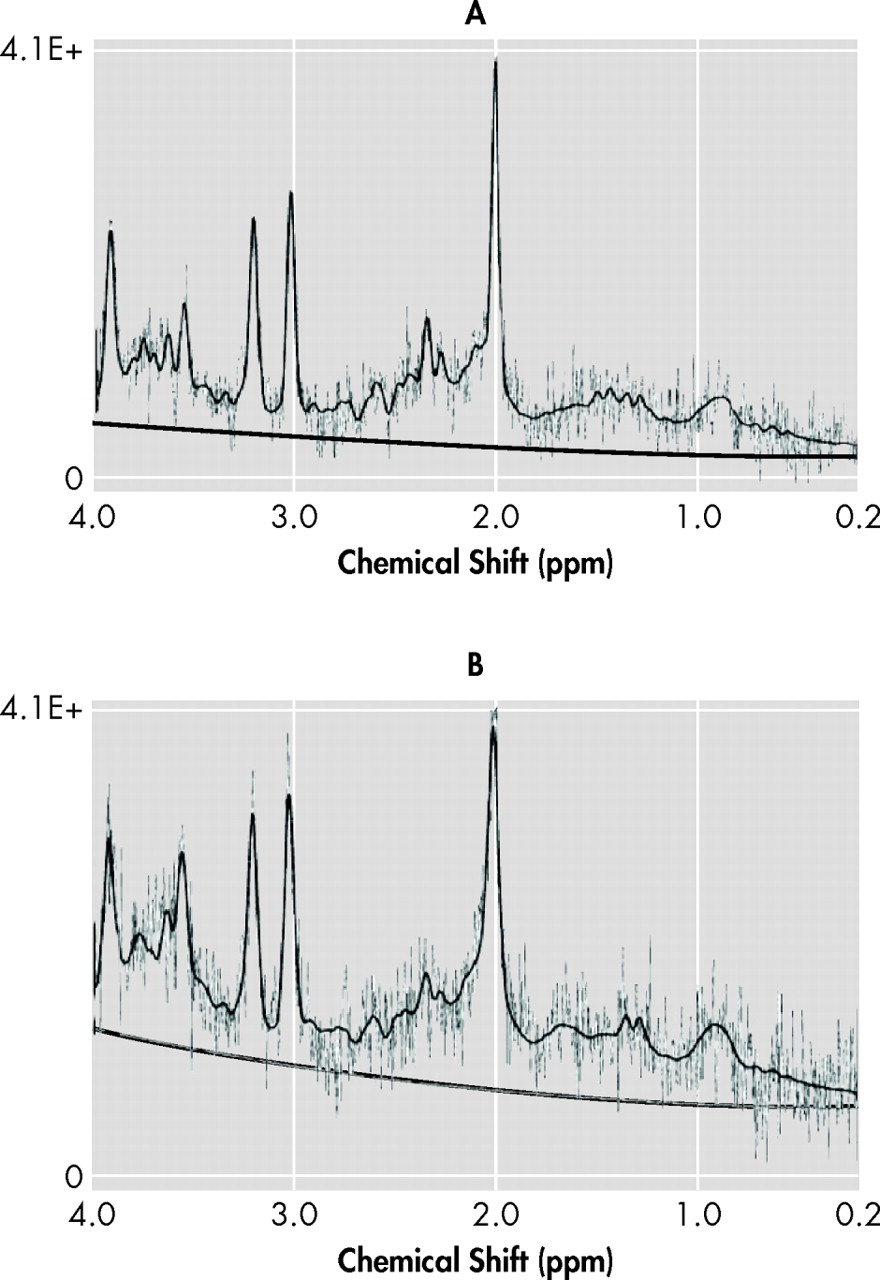
8 Proton MR spectroscopy (
1 H-MRS) detects signals from relevant brain metabolites such as
N -acetylaspartate (NAA), a putative neuronal marker
9 ; lactate; creatine; choline;
myo -inositol; and glutamate.
1 H-MRS conveys information independently from MRI signal changes, showing metabolic abnormalities in TBI beyond MRI-defined lesions.
10 Furthermore,
1 H-MRS shows loss of NAA in normal appearing white matter in TBI, which correlates with outcome.
10 –
12 Although most imaging studies have focused on recent TBI, less is known about long-term effects of TBI.
13The high incidence of psychiatric impairments in TBI survivors
2 and the selective hippocampal vulnerability in TBI
6,
7 can be reconciled within the conceptual frame of the limbic-cortical network model of mood regulation.
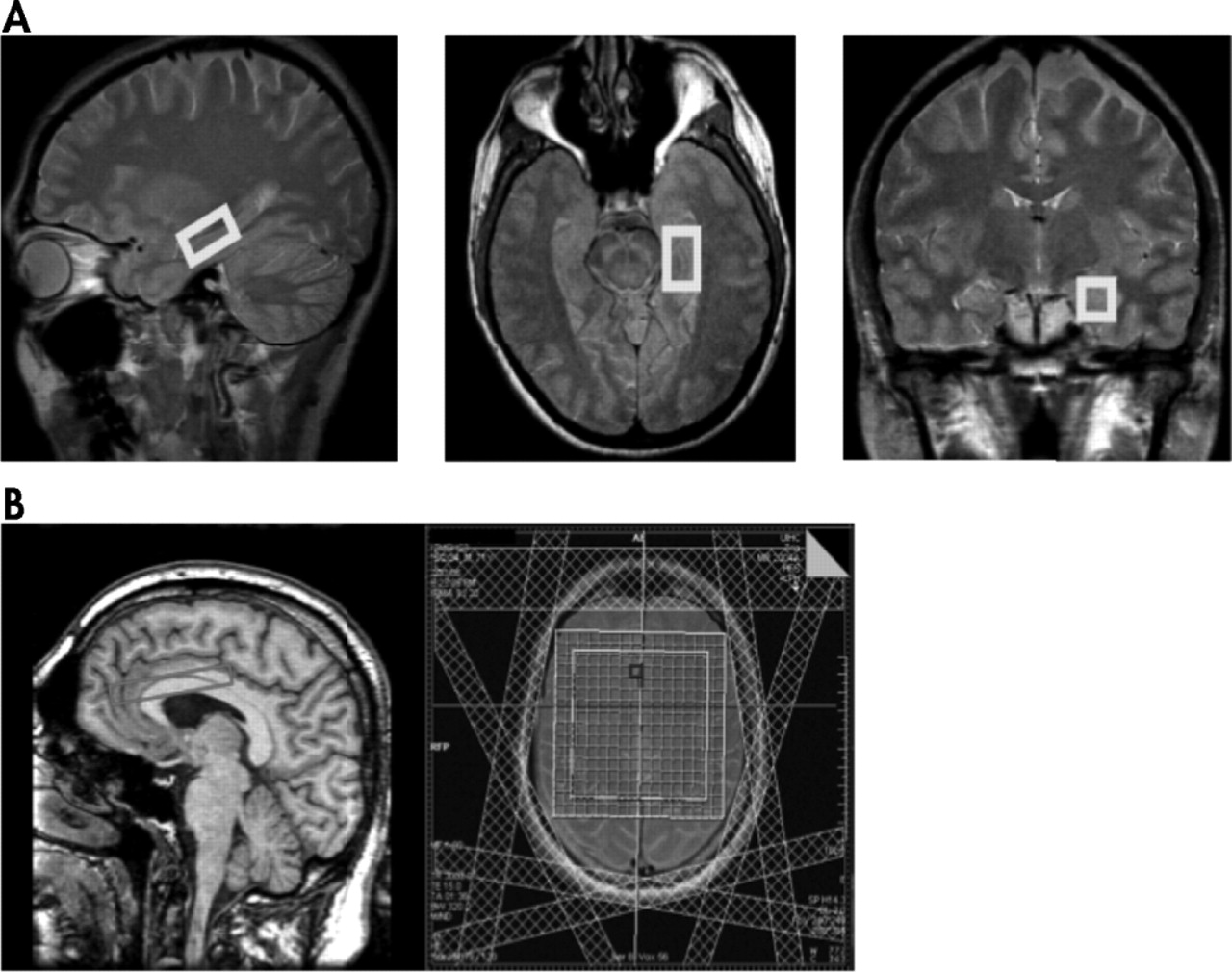
14 The purpose of this study was to quantify brain metabolites using
1 H-MRS at 3 Tesla in the left hippocampus, the left and right anterior cingulate cortex (ACC) and adjacent white matter in TBI patients remotely from the traumatic episode relative to age-matched comparison subjects and to examine their clinical correlates. We hypothesized that the NAA signal would be reduced in the left hippocampus and the ACC of remote TBI patients relative to comparison subjects. A second hypothesis was that NAA would inversely correlate with severity of mood and cognitive impairments (i.e., more severe impairments would be associated with lower NAA metabolic ratio in the left hippocampus and the ACC).
DISCUSSION
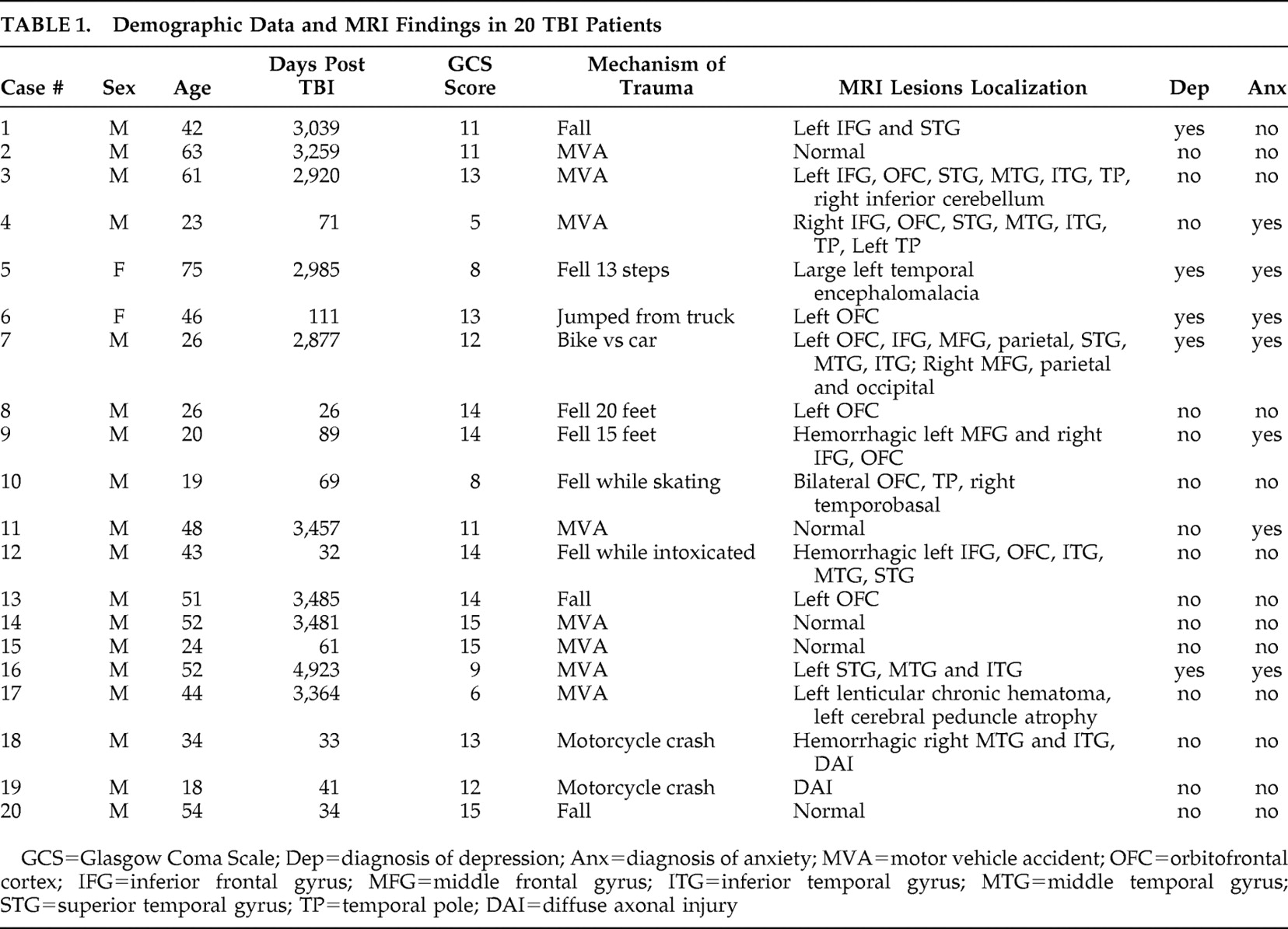
The first finding of this study was reduced tNAA to creatine ratio in the left hippocampus in remote TBI patients relative to age-matched healthy comparison subjects. Since only the left hippocampus was sampled with MRS for technical reasons, no assumptions on the laterality of hippocampal metabolic changes in TBI can be made. However, a possible explanation for the left-sided metabolic effects of both TBI (left hippocampus) and depression (left ACC) is that the patient sample had a significantly higher representation of left-sided MRI lesions (55%) than right-sided lesions (10%). Hippocampal neurons are particularly vulnerable to TBI, even when the impact site is remote from the medial temporal lobe. Stereological estimation of neuronal and glial cell numbers in a rat hippocampus after fluid percussion injury showed selective neuronal (but not glial) cell loss in the hilus and CA3.
21 Our finding of reduced tNAA to creatine ratio in the left hippocampus might reflect neuronal loss since NAA is a selective neuronal marker.
9 Therefore, reduction of neuronal NAA signal relative to nonspecific creatine probably reflects selective neuronal loss or metabolic impairment. Concomitant creatine increase, however, cannot be excluded while using ratios. Metabolic ratios are theoretically independent from atrophy, since the atrophy factor enters both the numerator and the denominator, thus being cancelled out (neglecting chemical shift). Moreover, when comparing left hippocampal tNAA to creatine ratio between patients with normal MRI (n=5) and those with lesions seen on MRI (n=15), no significant differences were found (mean=1.25 [SD=0.26] compared with mean=1.32 [SD=0.20], respectively; p>0.6). This further supports the notion that hippocampal metabolic abnormalities are independent from structural lesions.
Since the comparison group consisted of nondepressed healthy individuals, hippocampal NAA loss could be partially due to depression and not TBI. However, although limited by the small sample size, our analysis of depressed compared with nondepressed patients demonstrated mood-related changes in the left ACC, not in the hippocampus, suggesting that the reduced tNAA to creatine ratio in the left hippocampus was secondary to TBI and not depression. tNAA/choline was also reduced in the left medial temporal lobe using an 8 cm
3 voxel including the hippocampus in 20 chronic TBI patients relative to comparison subjects.
22 On the other hand, we did not detect significant NAA loss between TBI patients and comparison subjects in the left and right ACC and adjacent frontal white matter. Selective hippocampal vulnerability has been substantiated on injury models including in vitro
7 and in vivo
6 animal TBI paradigms. Hilar and CA3 neurons as well as newborn neurons of the dentate gyrus
6 are preferentially lost after trauma over neocortical neurons. Hippocampus and fornix atrophy has also been demonstrated after human TBI on imaging studies.
23,
24A second finding was that the subgroup of patients with a diagnosis of major depression had reduced tNAA to creatine ratio in the left ACC compared with nondepressed patients, independently from age and GCS score but not from time after trauma. These results are considered preliminary given the low statistical power secondary to the small sample size of depressed patients (n=5). Left ACC tNAA to creatine ratio reduction is theoretically independent from atrophy. In a previous TBI series there was an association of left frontal and basal ganglia lesions seen on CT scans with major depression.
25 Recently, postconcussion depressive symptoms in male athletes were correlated with gray matter volume in the right ACC.
26 Rostral cingulate metabolism has been proposed as a critical link in a limbic-cortical network model of depression.
14 Therefore, left ACC neuronal impairment as measured through the NAA signal may express dysfunction of a neuronal network underlying secondary mood disorders in TBI. Spectroscopic ACC alterations could then represent changes related to mood disorder and not to TBI since no spectroscopic differences were detected in the ACC between TBI patients and comparison subjects. A possible explanation for the lateralizing effect of depression on left ACC tNAA to creatine ratio, as noted above, is that our patient sample had a significantly higher representation of left-sided than right-sided MRI lesions. Actually, all five of our depressed patients had left frontal and/or temporal lesions on MRI. On the other hand, no metabolic abnormalities were recognized between patients with and without a diagnosis of anxiety disorder.
The third finding was the significant correlation between left hippocampal tNAA to creatine ratio and SFE scores. The instrument evaluates factors such as personal satisfaction with closest other relationship, vocational status, and ability of the family to cope with chronic illness, which are critical for quality of life and return to productivity after TBI. The reliability and validity of this instrument in patients with brain injury has been demonstrated.
27 Interestingly, the predictive role of left hippocampal tNAA to creatine ratio on psychosocial adjustment was independent from trauma severity, as measured with the GCS on admission, and patient’s age. Our data suggest that hippocampal neuron loss or dysfunction underlies poor behavioral and social outcome of TBI patients. This finding can be interpreted in light of the known deleterious consequences of left hippocampal injury in the cognitive and affective domains. In a previous study, for example, hippocampal atrophy was reported in TBI patients with mood disorders relative to severity-matched TBI patients without mood disorders.
8 Mood disorders, particularly major depression, are frequent complications of TBI and have a negative impact on patient recovery and functional outcome.
2 Finally, when comparing patients with normal and abnormal MRI, no significant differences in SFE scores were obtained (mean=0.16 [SD=0.09] compared with mean=0.15 [SD=0.17], respectively; p>0.8). Further analysis breaking down the MRI lesion group was not done given the heterogeneity of the lesions and the small sample size.
There were no significant correlations between RBANS total or domain specific scores and hippocampal or ACC metabolites. Previous studies have not found a consistent relationship between metabolites and neuropsychological scores. For instance, medial temporal lobe NAA/choline showed weak correlation with neuropsychological tests in TBI.
22 However, neurobehavioral MRS studies of the frontal lobes after childhood TBI demonstrated correlations with executive and social functioning.
28 Our neuropsychiatric metabolic correlations are exploratory in nature since no
a priori hypotheses or multiple comparison corrections were formulated.
An unexpected finding was reduced choline in the left frontal white matter of TBI patients. As shown in Table 1, 11/20 patients (55%) had left frontal injury based on structural MRI. Even though MRI lesions were avoided at the time of voxel selection, metabolic abnormalities of the normal appearing white matter may account for the reduced choline peak. A number of studies showed increased choline during the early phase and up to 1 year after TBI,
12 with a tendency to normalize on follow-up. NAA loss in the frontal lobes is also a dynamic process, with recovery in patients with good outcome and persistent low levels in patients with poor outcome.
29 Our finding of reduced left frontal white matter choline was associated with a nonsignificant trend toward reduction of the remaining metabolite peaks. Increased water content, which was used as the internal signal standard, in the preferentially injured left frontal lobe might account for these metabolic changes.
A number of limitations of this study have to be pointed out. The sample size was relatively small, particularly for the depressed TBI subgroup. The cross-sectional design of the imaging portion precluded analysis of follow-up data. Furthermore, patient sample heterogeneity resulted from inclusion of younger patients with recent trauma as well as older patients with remote TBI. Since noninjured comparison subjects were recruited instead of orthopedic trauma comparison subjects, secondary mood disorders could have contributed to the findings. On the technical side, the lack of coregistration of MR spectroscopy with segmented MRI obligated us to use metabolic ratios to reduce partial volume effects in cortical voxels. Finally, neuropsychological correlations are exploratory because no correction for multiple comparisons was performed.