Sepsis and its complications are the most frequent causes of morbidity and mortality in intensive care units (ICUs), contributing to 750,000 cases per year in the United States.
5 Survivors are often left with long-term cognitive impairments that can be disabling. Epidemiologic studies indicate that sepsis occurs in approximately 2% of all hospitalizations and may be present in up to 75% of ICU patients.
6 The long-term mortality rate after intensive care ranged from 50% to 60%.
7 The implementation of the surviving-sepsis guidelines was found to be associated with a significant decrease in mortality, with reports of in-hospital mortality being reduced from 57% to 37.5%.
8,9 Although the incidence and mortality rate of sepsis in children are lower than those in adults, sepsis is one of the leading causes of death in children.
10 One study found that the presence of encephalopathy with sepsis was associated with a twofold increase in the risk of death.
11 Sepsis-associated encephalopathy (SAE) is a multifactorial syndrome, characterized as diffuse cerebral dysfunction that results in an abrupt onset of impairment in cognitive functioning in the setting of sepsis. The hallmark clinical sign of SAE is acute altered mental status.
The various clinical manifestations that can present in the acute setting include inattention, disorientation, and agitation. SAE can also lead to stupor and coma. This syndrome has been reported to occur in 8%–70% of septic patients and is the most common encephalopathy in the ICU.
12 There is increasing evidence that SAE may pose substantial risks for long-term cognitive impairments, including alterations in mental processing-speed, executive function, memory, attention, and visual-spatial abilities. These may last for several years after an admitting diagnosis of SAE, affecting functional abilities, quality of life, and the ability to return to work. Depending on the degree of impairment (e.g., mild, moderate, or severe), there may also be a tremendous burden placed on both family members and caregivers. Recent studies have concluded that 70% of sepsis survivors had neurocognitive impairments at hospital discharge, and up to 45% had neurocognitive impairments at 1 year.
13 Survivors of critical illness are also likely to experience significant symptoms of anxiety and depression.
14Many patients who have been diagnosed with SAE have chronic neurocognitive sequelae up to 6 years after discharge from an ICU.
15 Impairment was generally diffuse, but occurred primarily in areas of psychomotor speed, visual and working memory, verbal fluency, and visual construction. The rate of neuropsychological deficits in this study population was markedly higher than population norms for mild dementia. The odds of developing moderate-to-severe cognitive impairment were 3.3 times higher after an episode of sepsis.
16 Extrapolating from national data, the authors estimated that sepsis may contribute to 20,000 new cases of moderate or severe cognitive impairment in the United States each year. A small case series found that, 3 months after release from an ICU, all the patients demonstrated severe memory, executive-function, and attentional impairments.
17 Those who survived past hospitalizations for severe sepsis were also found at follow-up to have new functional limitations.
16 These limitations occurred in performing basic tasks of everyday life, such as eating and bathing, and instrumental activities for daily living related to independence. Another study found that 44% of children surviving septic shock had cognitive scores <25% of the norm population, and 14% of the children attended special-education schools.
18 Other significant neuropsychiatric symptoms that ICU survivors present are depression and anxiety. The prevalence and the severity of the affected patients vary from 10% to 58%.
19 Depression was reported in up to 30% of the survivors, and 47% were reported to have clinically significant anxiety.
20Delirium is an independent risk factor for long-term cognitive impairment in patients without sepsis, and it is a term that is also used to describe patients with SAE. It is characterized as an acute impairment of cognition, with a fluctuating course, impaired attention, and altered level of consciousness. The incidence is as high as 82% among mechanically-ventilated patients.
21 Delirium is associated with several adverse outcomes, including increased morbidity and mortality, prolonged hospitalization, and poor surgical outcome.
22 The duration of delirium in mechanically-ventilated patients is a predictor of cognitive impairment (as measured by neuropsychological testing) at both 3 and 12 months after hospital discharge.
23Sepsis is defined as a syndrome that is characterized by the presence of both infection and a systemic inflammatory reaction. It represents an imbalance between pro-inflammatory and anti-inflammatory response to a pathogen.
24 Sepsis results when an inflammatory response to infection becomes generalized, and extends to involve normal tissue remote from the initial site of injury. This previously healthy tissue will display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. These manifestations of inflammation are recognized as the systemic inflammatory response syndrome (SIRs), which refers to the consequences of a deregulated host inflammatory response. It is clinically recognized by the presence of two or more of the following: elevated temperature, tachycardia, tachypnea, and leukocytosis/leukopenia.
25 The pathogenesis of sepsis revolves around several mediators. These include tumor necrosis factor alpha (TNF-α), interleukins, platelet-activating factor, leukotrienes, thromboxane A2, and activators of the complement cascade.
26,27 The result is a cascade of events that lead to end-organ and cellular damage. Autopsy studies have shown widespread endothelial and parenchymal cell injury. Possible mechanisms include ischemia, direct cell injury, and increased rate of apoptosis. Recent data suggest that the pathophysiology underlying sepsis may not only be related to an overactive immune system, but may also indicate an immune system that is severely compromised.
28 The pathophysiology of SAE is unclear, but it is most likely related to release of inflammatory mediators. There is evidence of blood–brain barrier dysfunction in both patients and rodent models of sepsis.
29 Pulmonary, cardiovascular, renal, and gastrointestinal dysfunction have also been characterized in sepsis-induced multiple organ failure, which is associated with a high mortality rate in humans.
30 Cecal ligation and perforation is one of the widely used murine models of sepsis. This induces a polymicrobial sepsis that mimics human sepsis, and has contributed to the understanding of the pathogenesis of SAE.
31On the basis of animal models, it is believed that microcirculatory alterations, disturbance of cerebral autoregulation, damage to the blood–brain barrier, and the direct effect of the inflammatory process on glial cells play a decisive role. This model is able to mimic clinical SAE because it induces both autonomic dysfunction and long-term cognitive impairment.
32 Endotoxin, a lipopolysaccharide, is implicated in the pathogenesis of sepsis and is well established as a trigger for the cellular and pathophysiological response to sepsis.
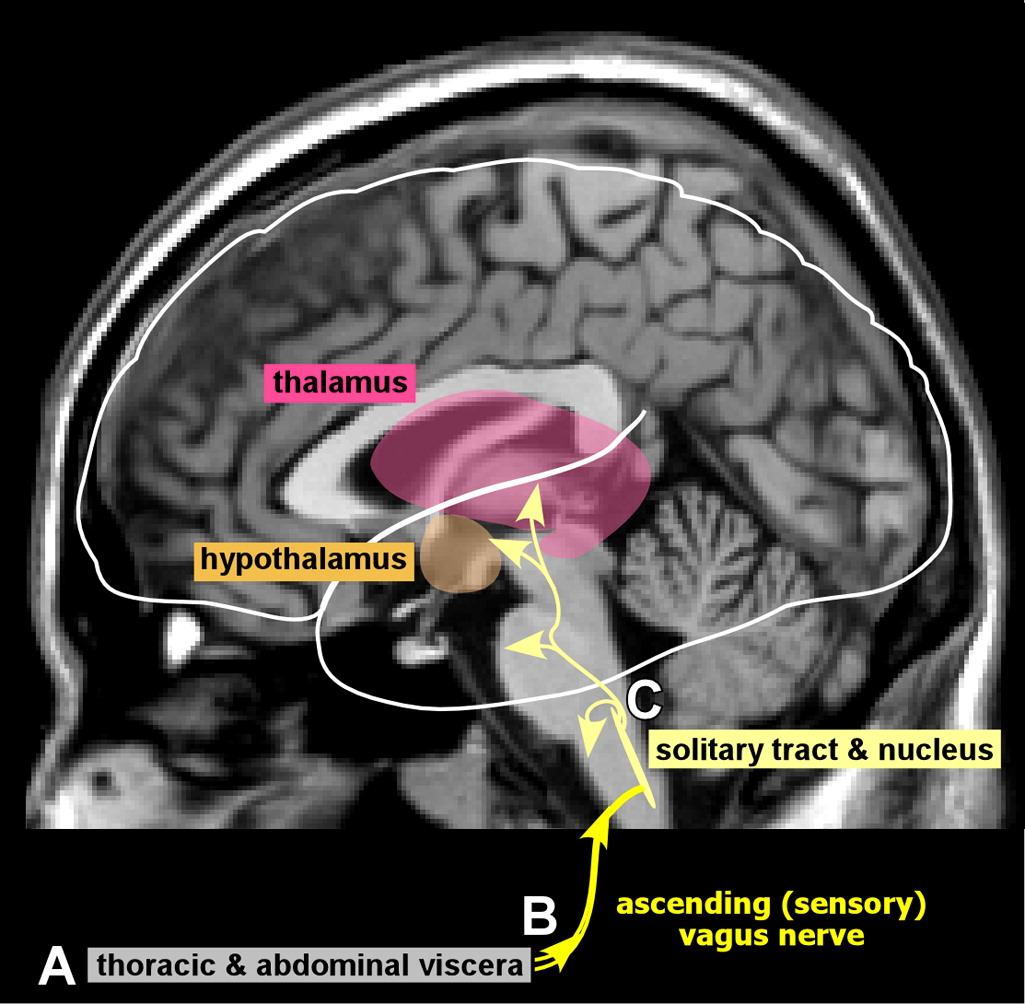
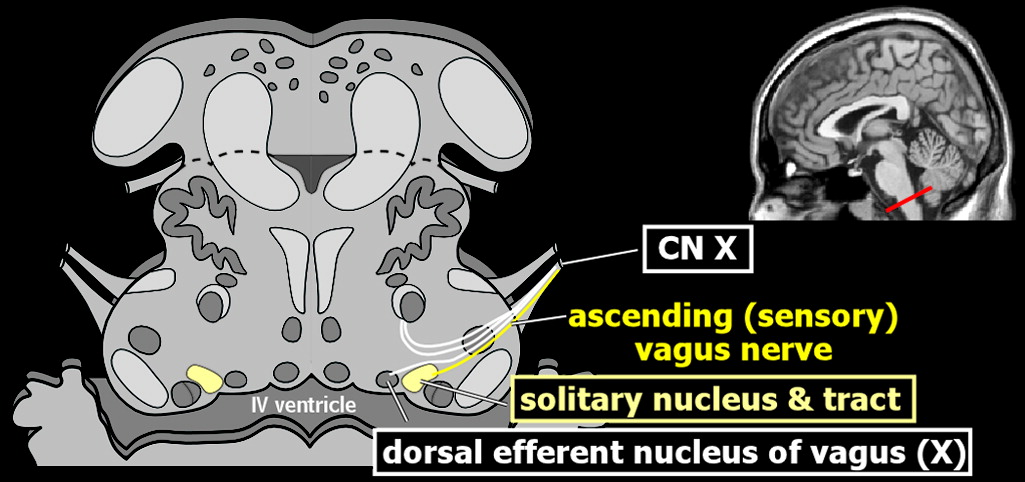
33 The process is thought to be initiated when the brain first senses systemic inflammation. During this initial crucial step, the blood–brain barrier is intact and opposes entrance of circulating inflammatory cells. The vagus nerve and peripheral organs are the conduits by which peripheral manifestations of systemic inflammation are relayed to the brain.
1,2 It is the peripheral organs that contain the specific markers for inflammation (e.g., interleukin-beta, tumor necrosis factor) that are powerful inducers of the inflammatory response.
34 These substances stimulate the afferent vagus nerve, which signals detection of inflammation centrally (
Figure 1).
1,2 As a consequence, glial cells begin to express both immune receptors and mediators (e.g., cytokines, nitric oxide synthase, prostaglandins), which are thought to be the counter-regulatory response. It is the activation of cerebral endothelial cells that results in the breakdown of the blood–brain barrier. The increased permeability of the blood–brain barrier has been documented in experimental animal models.
12 Cerebral activation of the endothelial cells also alters the microcirculation and vascular tone, changes that can lead to ischemia or hemorrhagic lesions.
35 The mediators are directly involved in the release of neurotransmitters (e.g., GABA, ACh) and neurohormones (e.g., ACTH, vasopressin).
The formation of reactive oxygen species compromises cell function and survival. Oxidative stress plays a major role by inducing cell apoptosis. This stress response is induced by nitric oxide, which has been correlated with neuronal and microglial apoptosis. Development of cytotoxic edema (cortex and hippocampus), vasogenic edema (base of brain), and neuronal injury were documented with MRI and spectroscopy after septic challenge in the mouse.
36 Studies of sepsis in another animal model (rat) have reported an oxidative stress response in the hippocampus and cortex, and increased apoptosis in hippocampus, dentate gyrus, median preoptic nucleus, and the subventricular zone.
37,38The diagnosis of SAE may be difficult to ascertain using the clinical evaluation alone.
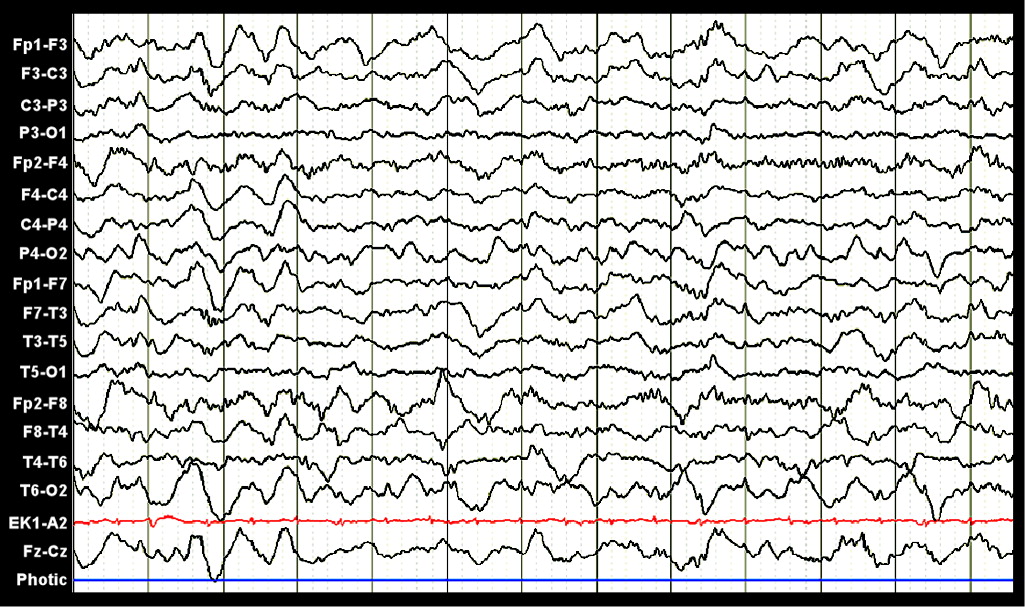
39 The physiologic response of the brain can now be monitored with use of both continuous EEG (cEEG) and transcranial Doppler. These modalities are extremely helpful in the unstable patient, as they can be performed at the bedside. A recent study found that 22% of patients with no history of previous neurologic injury who were placed on cEEG monitoring demonstrated both periodic epileptiform discharges and seizure activity.
40 In two-thirds of cases, these EEG abnormalities did not display any clinical accompaniment. Electrographic seizures were recorded in 10% of cases, and septic patients were at higher risk of demonstrating either electrographic seizures or periodic epileptiform discharges. Both types of EEG abnormality were found to be associated with death or severe disability at discharge. The overall most frequent EEG pattern found in medical ICU patients is generalized slowing (
Figure 2).
41Sepsis-associated encephalopathy is one of the many disorders that is thought to impair the vasodilatory response of the cerebrum. Transcranial Doppler is used to assess cerebral vasomotor reactivity. This is defined as the vasodilation capacity of cerebral arterioles to external stimuli, such as increasing extracellular pCO
2 and decreasing extracellular pH. Thus, it provides information regarding cerebral autoregulation (the brain's inherent ability to maintain a constant blood flow over a wide range of cerebral perfusion pressures) and collateral circulation.
42 Acetazolamide, the reversible inhibitor of the enzyme carbonic anhydrase, has been used to test cerebral vasomotor reactivity in various diseases and conditions. It induces a slight temporary hypercapnia lasting for approximately 20 minutes, which results in vasodilation of the cerebral arterioles, most probably through inducing nitric oxide synthesis. Dilation of these vessels results in a decrease of cerebrovascular resistance. In one series of septic patients without hemodynamic compromise or need of hemodynamic support, the ability of the cerebral arterioles to dilate was decreased, as measured by blood-flow velocities.
43 Brain histopathology in septic shock reveals blood–brain barrier disruption, cerebral edema, microglial activation, cerebral infarcts, hemorrhages, intravascular thrombosis, microabscesses, multifocal necrotizing leukoencephalopathy, and neuronal cell death.
44 Another autopsy study found evidence of hypoxic ischemic injury involving primarily the hippocampus, pons, and striatum.
45 The use of brain imaging in this patient population may guide treatment and care as well as characterize any lesions.
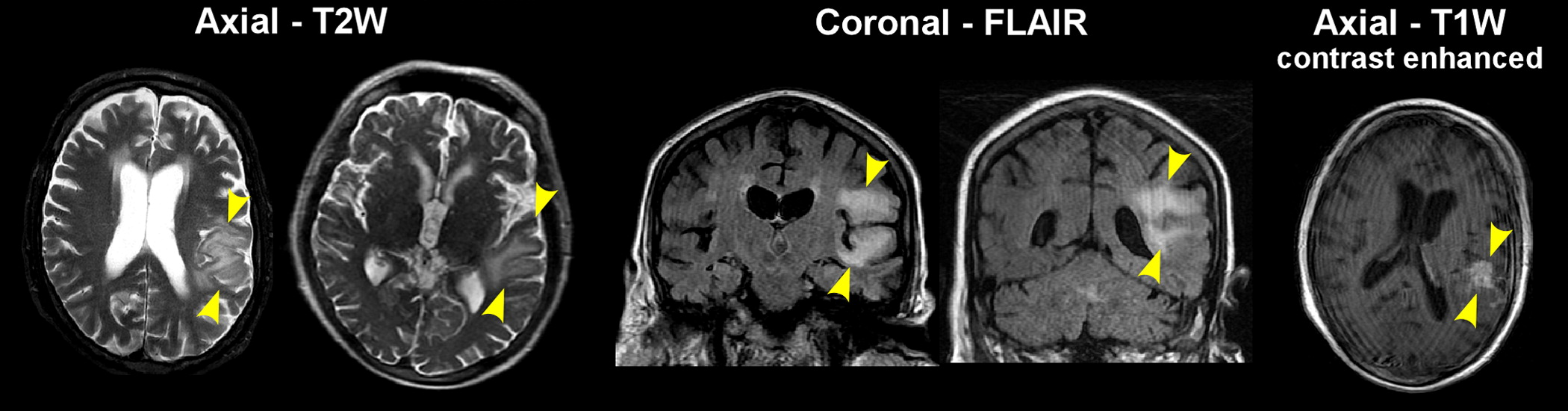
There are few reports regarding brain imaging in patients with SAE. Two recent small prospective studies of SAE reported abnormal imaging findings in the majority of patients.
46,47 Lesions in the subcortical white matter, with imaging appearance consistent with vasogenic edema (e.g., increased signal on T2-weighted imaging, decreased signal on diffusion-weighted imaging, increased apparent diffusion constant), was the most common presentation (
Figure 2). Size and number of lesions varied considerably across patients. In one study, all patients who eventually died had abnormal imaging.
46 Imaging in related conditions may also be informative. Posterior reversible encephalopathy syndrome (PRES) is a clinical radiographic syndrome characterized by headache, confusion, seizures, and visual loss.
48 It is associated with white-matter edema primarily affecting the posterior occipital and parietal lobes in a variety of conditions, including hypertension and immunosuppression. PRES was associated with infection or sepsis in 7%–24% of patients in two recent retrospective studies.
49,50 A neuroimaging case series of eight ICU patients who were diagnosed with delirium (no focal neurological findings) found white-matter hyperintensities in six and mild atrophy in one.
17 No patient had ischemic/hemorrhagic lesions, and neuroimaging in this small case series did not lead to new diagnoses or immediate changes in therapy. A retrospective study of ICU patients who underwent neuroimaging because of neurological changes such as alteration in mental status, confusion, delirium, or coma, reported imaging abnormalities in 64% (41/64), including seven in which the initial neuroimaging examination was normal.
51 The most common abnormalities were atrophy and white-matter hyperintensities. Patients with and without neuroimaging findings did not differ in age, length of hospitalization, time in ICU, or outcome. Risks and benefits must be weighed before obtaining any brain imaging. The use of MRI in comparison with CT affords a more detailed illustration of the brain, more specifically, the white matter. Its use is limited in the unstable patient, given the duration to complete the study, and a head CT offers a better alternative in these cases.
In conclusion, sepsis remains a frequent cause of morbidity and mortality. SAE is the most common type of encephalopathy that is seen within a medical ICU. The underlying pathophysiology is still not fully understood. Survivors may have neurocognitive sequelae for months-to-years after discharge. Anxiety and depression are also common neuropsychiatric presentations in this patient population after discharge. There are few imaging studies in SAE, and more research in this area is needed to further aid in prognosis. One needs to consider the possibility of sepsis-associated encephalopathy in the differential diagnosis for both neurocognitive impairment and new-onset psychiatric disorders.