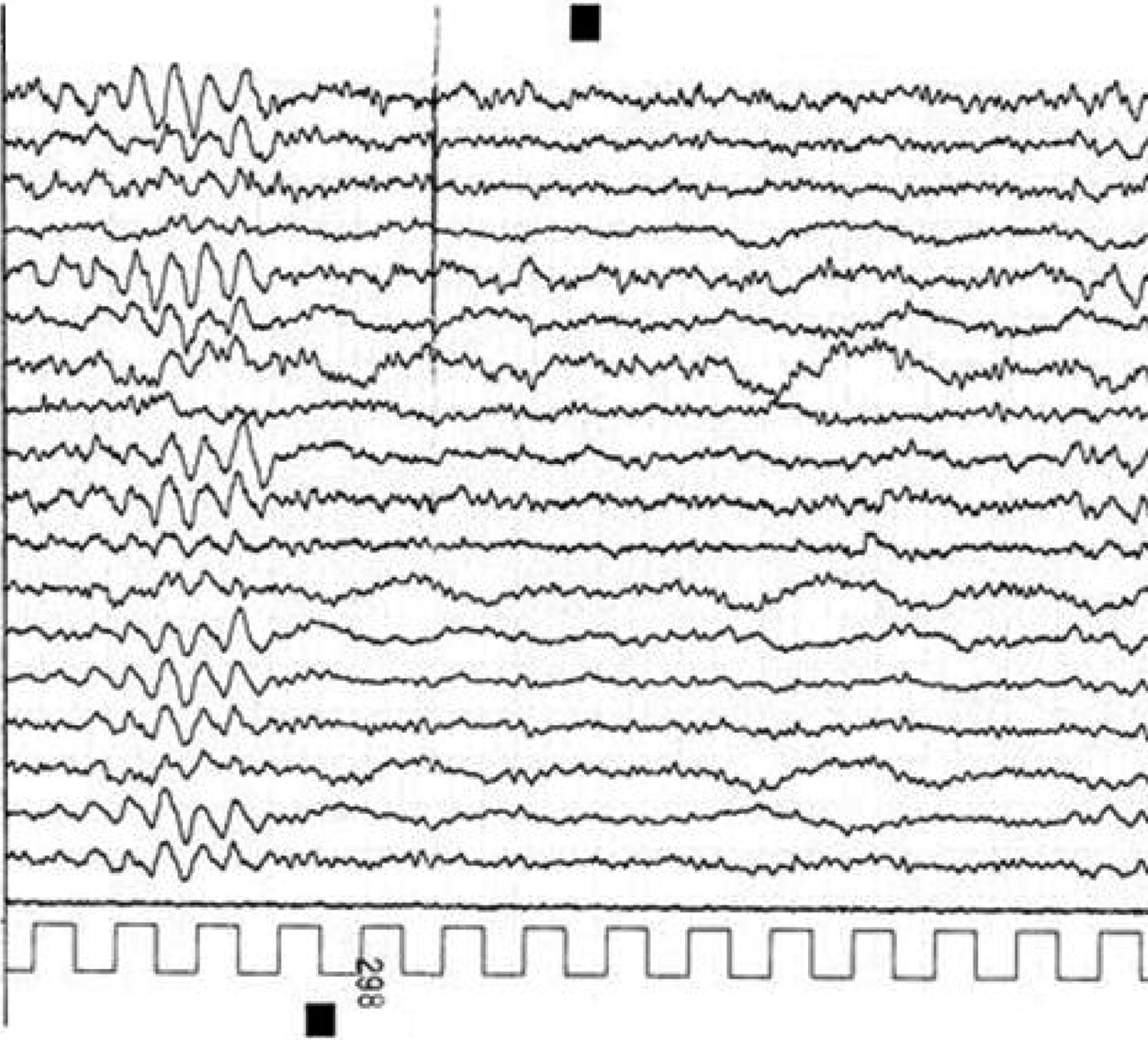
Although usually well tolerated, carbamazepine is known to induce many neurological adverse effects, including tremor, somnolence, ataxia, nystagmus, and worsening of preexisting epileptic syndromes.
1 These reactions may occur with toxic drug levels and even within the normal therapeutic range. The underlying pathophysiology of these phenomena is still unclear, and an idiosyncratic reaction should also be considered. In the majority of cases, the encephalopathy is acute and reversible, and CBZ withdrawal leads to a normalization of the clinical and EEG picture. In our patient, there was a clear temporal relationship between drug administration, drug serum levels (above normal range), and the development of the encephalopathy. The causative effect of CBZ is confirmed by rapid recovery, after drug discontinuation, of both clinical and EEG abnormalities. CBZ occasionally induces EEG changes even in seizure-free patients. The predominant, associated EEG findings are a diffuse slowing of background activity, the development of focal slow-wave activity, and intensification of secondary bilateral synchrony. Burst suppression pattern, pseudo-periodic sharp-wave activity, and alpha-coma pattern have also been observed during CBZ intoxication.
2 In our case, EEG findings consisted predominantly of FIRDA occurring on a diffuse, slowing background. To the best of our knowledge, a similar pattern has never been described during adult CBZ encephalopathy. “There is a single report of 16-year-old girl with a large, toxic overdose of carbamazepine, in which EEG was dominated by occipital delta activity (OIRDA), because of which authors hypothesized brainstem disorders.
3 FIRDA was originally described by Cobb
4 in 1945. It is a rhythmic slow-wave discharge localized in the frontal regions, with a frequency of 1.5 to 4.0 c/sec. Although the pathophysiological significance of FIRDA is unknown, it may reflect many pathological conditions, such as deep midline lesions and posterior fossa tumors.
5 However, it has also been reported in association with subcortical lesions, hydrocephalus, cerebral edema, increased intracranial pressure, and acute basilar migraine.
6FIRDA is often observed in metabolic encephalopathy, especially in patients with diffuse or focal brain lesions.
7 For example, FIRDA often occur in dialytic encephalopathy in subjects with chronic renal failure. Moreover, the association between FIRDA and metabolic dysfunction is still unclear. More recently, several reports have correlated FIRDA with long-term medication, especially antipsychotic agents.
8 In such cases, EEG abnormalities occur in a dose-dependent manner and are predominantly observed in schizophrenic patients receiving high daily dosages of antipsychotic drugs. On the other hand, FIRDA are quite common in elderly patients with partial epilepsy and with cerebrovascular disorders. In a study by Fariello, 44% of patients with FIRDA had focal structural lesions; 34%, diffuse structural; and 22% had nonstructural disease.
9 Brain MRI revealed small lacunae, mostly involving the basal ganglia, whereas our patient's EEG recording was normal before CBZ treatment; thus, a clear relationship between a toxic level of CBZ and FIRDA is seen in the case of our patient. Our evidence suggests that a toxic CBZ dose can trigger EEG abnormalities in a patient with structural brain lesions, even if mild. We conclude that patients with chronic, mostly ischemic brain lesions may be predisposed to develop FIRDA during acute CBZ encephalopathy, as well as during acute metabolic derangement.