Case Report
A 94-year-old woman described an acute onset of visual hallucinations such as prosopometamorphopsia (facial distortion) and macropsias. For example, when she saw her son, he had first appeared normal to her and then suddenly seemed to convert into a giant with a distorted body. In other episodes, she suddenly saw skyscrapers or strange figures passing by. The events mostly lasted several minutes and frightened her very much. There were no additional sensory phenomena such as acoustic or olfactory hallucinations. Although these visual sensations frightened her, she was fully aware that they were not real. The remainder of the clinical neurological examination was unremarkable; specifically, there were no sensory or motor deficits.
In her medical history, the woman had had a myocardial infarction several years ago, a hyperlipoproteinemia, and a mild renal insufficiency, as well as multiple arthritic complaints appropriate to her age. She did not suffer from dementia or any other psychiatric disorder.
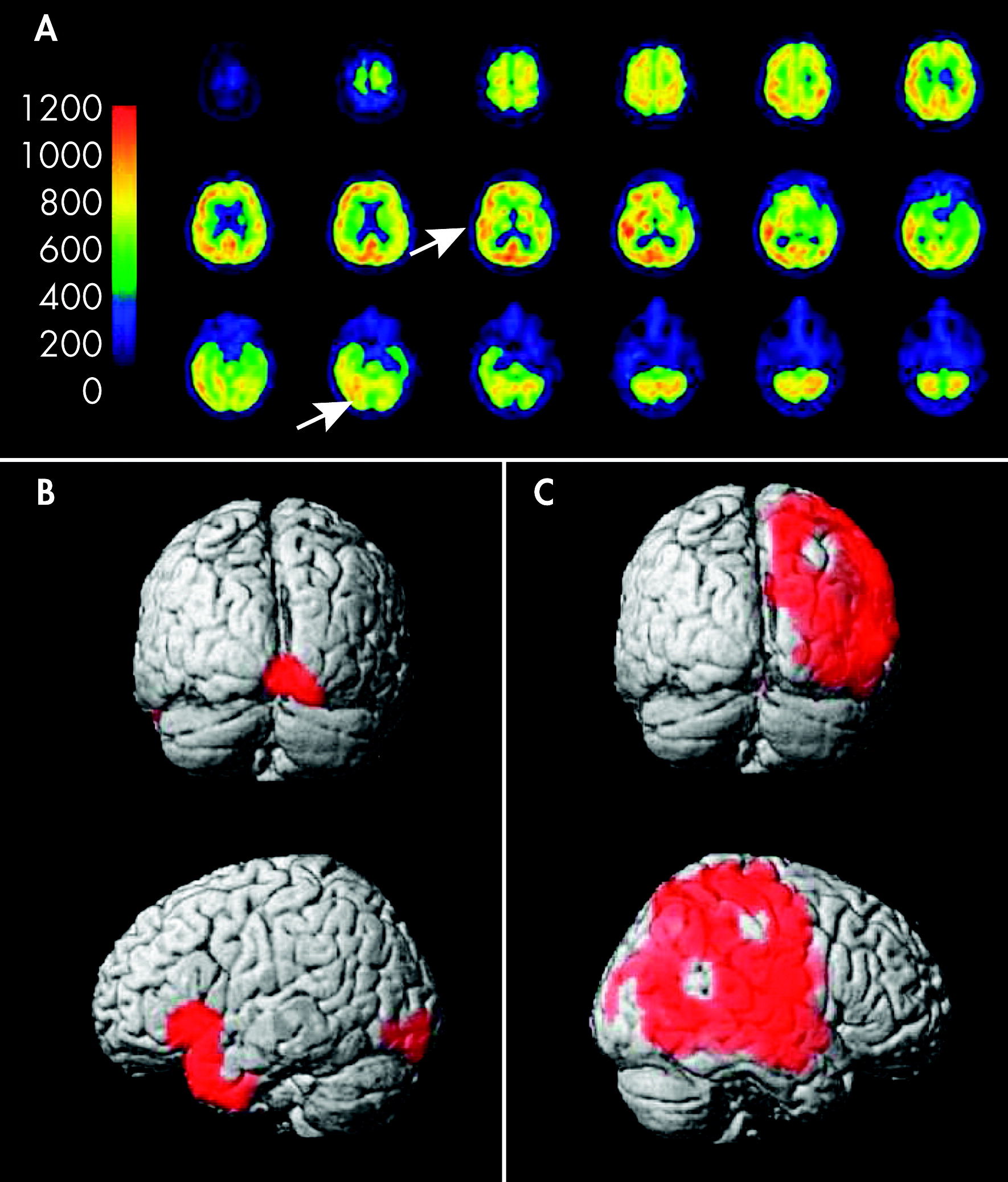
An MRI scan of the brain showed a mild vasculopathic encephalopathy, but no acute ischemic lesion. An ophthalmologic examination detected a mild glaucomatic optic atrophy and a senile cataract. An electroencephalography (EEG) did not show any focal nor epileptiform discharges. At this time, she still had frequent episodes of hallucinations. For further investigation, we performed a quantitative FDG-PET of the brain. This showed a global absolute diminishment of mean metabolic rate of glucose in the brain and also a local relative diminishment of glucose uptake in the right occipital area (dorsal middle and inferior occipital gyrus) corresponding to the primary visual cortex (V1–2). In the adjacent visual associative area (ventral middle and inferior occipital gyrus, superior occipital gyrus, dorsal temporal lobe, including V4–5), a local augmentation of glucose uptake was recognized (see statistical parametric maps in
Figure 1). These findings most likely represented the pathology responsible for the patient's visual hallucinations and the temporarily-occurring visual field defects. The patient was treated with topical antiglaucomatous eye drops (travoprost, prostaglandin analogue). The visual hallucinations quickly regressed, and the patient was discharged without symptoms.
Discussion
It has been proposed that the complex visual hallucinations in CBS arise from cortical visual association areas after deafferentiation from the primary visual cortex (“deafferentiation theory”).
1 The lack of sensory input to the associative cortex is proposed to cause spontaneous neuronal discharges leading to abnormal visual perceptions. Therefore, the symptoms can arise from damage in any part of the visual system between the eye (e.g., macular degeneration or glaucoma) and the primary visual cortex (e.g., occipital lobe infarction) by which a diminished sensory input into the association areas can result.
2In a recent study comparing low-vision subjects with normal-vision control subjects, visual hallucinations were observed in 34% of low-vision patients, indicating that CBS is quite common in patients with eye diseases. However, only a few patients spontaneously report these experiences to their doctors.
3 In most of these cases, symptoms were only mild and unformed; for example, some bright lights or spot-like images.
Nonetheless, the symptoms in our patient were rather severe, leading to emotional distress. Our patient also had low vision due to glaucomatic optic atrophy and a senile cataract. As a result, it might have caused a diminished sensory input to the primary visual cortex, resulting in a regional hypometabolism on one side. This, in turn, might have led to a deafferentiation of the visual associative cortex. In the performed MRI scans, no further explanations, such as damages to the visual cortex (e.g., infarction) were found. In SPECT examinations in patients with visual hallucinations after occipital lobe infarction, focal hypoperfusion in the occipital cortex has already been demonstrated.
4 In fMRI studies, an increased activity in visual association cortical areas already has been shown.
5In our patient, there was a hypometabolism in the primary visual cortex (V1–2), which was visualized by FDG-PET. Also, a hypermetabolism in the visual associative cortex (V4–5) was seen, which corresponded well with the complex visual hallucinations. Thus, the results of this examination support the deafferentiation theory: There is a lack of sensory input into the associative cortex as a result of a hypometabolism in the primary visual cortex and consecutive neuronal discharges in the associative cortex expressed by a hypermetabolism.
Acknowledgments
This study was not sponsored.
Dr. Garde is investigator in clinical studies which are sponsored by Biogen Idec, Sanofi Aventis, Bayer Health Care, Novartis and Lilly. Dr. Garde received funding for trips to national congresses from Biogen Idec, Teva/Sanofi Aventis and Baxter.
Dr. Skipuletz is investigator in clinical studies sponsored by Biogen Idec, Sanofi Aventis, Bayer Health Care, Novartis, and Lilly. Dr. Skripuletz received funding for trips to international and national congresses from Biogen Idec, Octapharma, and Bayer Health Care.
Dr. Pul is investigator in clinical studies sponsored by Biogen Idec, Sanofi Aventis, Bayer Health Care, Novartis, Glaxo Smith Kline, and Lilly. Dr. Pul received funding for trips to national congresses from Biogen Idec.
Research of Dr. Berding was funded by Bayer Health Care, Lundbeck, GE Health Care, and National Funds (BMBF).
Dr. Weissenborn received honoraria from serving on a scientific advisory board of Boehringer Ingelheim.
Dr. Trebst received honoraria for consulting and lectures from Bayer Health Care and received funding for trips to international and national congresses from Bayer Health Care, Merck Serono, Biogen Idec, and Sanofi Aventis. Dr. Trebst is investigator in clinical studies sponsored by Biogen Idec, Sanofi Aventis, Bayer Health Care, Novartis, Glaxo Smith Kline, and Lilly.