A clinical trial of an anticonvulsant in the treatment of bipolar disorder in children may be more notable for its publication than its outcomes.
The multicenter trial found that oxcarbazepine was “not significantly superior to placebo” in a seven-week test of youngsters between the ages of 7 and 18.
“Five or 10 years ago papers like this were routinely rejected because they showed `negative results,' “ Scott Zeger, Ph.D., professor and chair of biostatistics at the Johns Hopkins Bloomberg School of Public Health, told Psychiatric News. “This negative study is important both because it teaches us about this particular trial, but also because it reflects a trend in favor of publication of negative studies, allowing us to overcome biases that have been rampant in the medical literature.”
The study sought to fill a largely empty space in the research on treatment of bipolar disorder in young people, added Ellen Leibenluft, M.D., of the National Institute of Mental Health, in an editorial accompanying the trial's publication in the July American Journal of Psychiatry. The only previously published placebo-controlled trial of the drug in adults with bipolar disorder included only six patients.
“Parents and clinicians can only be appalled by the contrast between the ample evidence documenting the severity of pediatric bipolar disorder and the paucity of controlled trials to guide its treatment,” wrote Leibenluft.
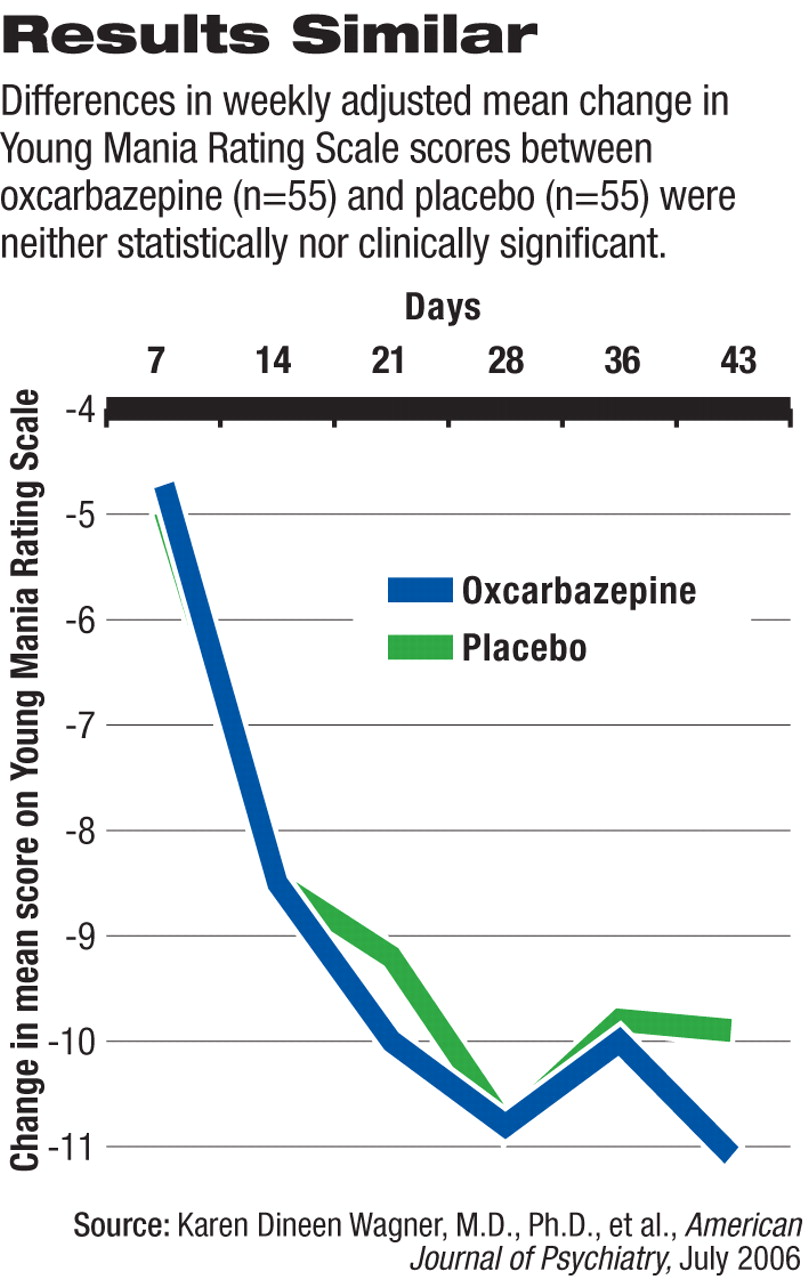
To fill this gap, karen Dineen Wagner, M.D., Ph.D., professor and vice chair in the Department of Psychiatry and Behavioral Sciences and director of the Division of Child and Adolescent Psychiatry at the University of Texas Medical Branch in Galveston, and colleagues recruited 116 outpatients who had been diagnosed with bipolar I disorder, manic or mixed episode, using DSM-IV criteria. The subjects also had a score of 20 or above on the Young Mania Rating Scale (YMRS) at the baseline visit. Half the patients were randomly assigned to get oxcarbazepine and half to get a placebo in the double-blind trial.
Fifty-five patients in each group were available for efficacy analysis. A large percentage of each group dropped out of the trial before its completion—20 patients (34 percent) in the oxcarbazepine group and 24 (42 percent) in the placebo arm. Patients received an average dose of 1,515 mg/day of oxcarbazepine for a median 48 days.
The study was supported by funding from Novartis Pharmaceuticals.
Mean YMRS scores dropped among both groups of patients, and no statistically significant differences were found at any point during the trial, the authors noted. The adjusted average change was -10.90 for the oxcarbazepine group and -9.79 for the placebo group.
Adverse Events Common
Most of the patients had at least one adverse event—90 percent of those taking oxcarbazepine and 79 percent of placebo patients. The oxcarbazepine group was more likely to experience dizziness, nausea, sleepiness, double vision, fatigue, or rash. The effects were mild to moderate, of short duration, and likely to occur in the early part of the study.
“The most important thing is that people are aware of all trials, whether they have a positive or negative outcome.”
About 42 percent of the patients in the oxcarbazepine arm and 26 percent in the placebo arm achieved the secondary efficacy goal of at least a 50 percent reduction in the YMRS score. There was no statistically significant difference between these figures, the researchers noted.
Leibenluft wrote in the accompanying editorial, “Without the placebo arm, in which 26 percent of patients responded, the authors might have concluded that a response rate of 42 percent demonstrated that oxcarbazepine is effective in treating pediatric bipolar disorder, when in fact there was no significant difference in outcome between the two treatment groups.”
Statistical vs. Clinical Significance
Even nonsignificant trends toward an effect can guide clinicians, said Zeger.
“Statistical significance is not the same as clinical significance,” he said. “Knowing how to quantify evidence in a trial is not the same as making a treatment decision.”
He continued, “The most important thing is that people are aware of all trials, whether they have a positive or negative outcome. This is a shift in position. Once, studies showing no difference in effect would have been called `failed trials.' Often they were self-censored by authors, who figured they weren't worth the effort to publish, or by journal editors who thought they weren't interesting to readers. As a result, readers would tend to think that treatments they did read about were better than they really were.”
“Although the results of the paper do not quite reach significance,” said Robert Freedman, M.D., the editor in chief of the American Journal of Psychiatry, “there are no comparable studies that attempt to provide empirical evidence to clinicians about the safety and efficacy of antiepileptic drugs for the treatment of bipolar disorder in adolescence and childhood. Therefore, the journal decided to publish these data with an accompanying editorial explaining their limitations.”
One way to track all clinical trials, regardless of their outcomes, is to register every trial when it begins in a recognized database, such as the National Institutes of Health's ClinicalTrials.gov.
The World Health Organization now recommends registration of all medical studies that test treatments on human beings. Editors of major medical journals (including the American Journal of Psychiatry) have declared that they will not publish trials that have not been registered and will adhere to standards set by the International Committee of Medical Journal Editors in 2004.