APA has expressed “extreme concern” over the lack of a therapeutic category for medications used to treat substance use disorders in the proposed revision of the United States Pharmacopeia's (USP) Model Guidelines for prescription drug plan (PDP) formularies participating in the Medicare Part D drug benefit.
Following provisions of the Medicare Modernization Act (MMA) that created the Part D drug benefit, USP was charged by the Centers for Medicare and Medicaid Services (CMS) to develop a uniform set of guidelines that CMS could use to evaluate individual PDPs' proposed formularies.
The MMA also specified that USP is to periodically update those model guidelines to ensure that they include a complete and accurate listing of the drug categories, classes, and key drug types that all Part D formularies must cover (see
page 1). USP recently released its proposed revisions for the Model Guidelines to be used in 2007 and invited public comment, as required by the MMA.
In a letter signed by APA President Steven Sharfstein, M.D.; Michael Gendel, M.D., president of the American Academy of Addiction Psychiatry; Elizabeth Howell, M.D., president of the American Society of Addiction Medicine; and Michael Brooks, D.O., president of the American Osteopathic Academy of Addiction Medicine, the four organizations told USP that a specific category should be added to the model guidelines to encompass the FDA-approved medications currently used to treat addictions.
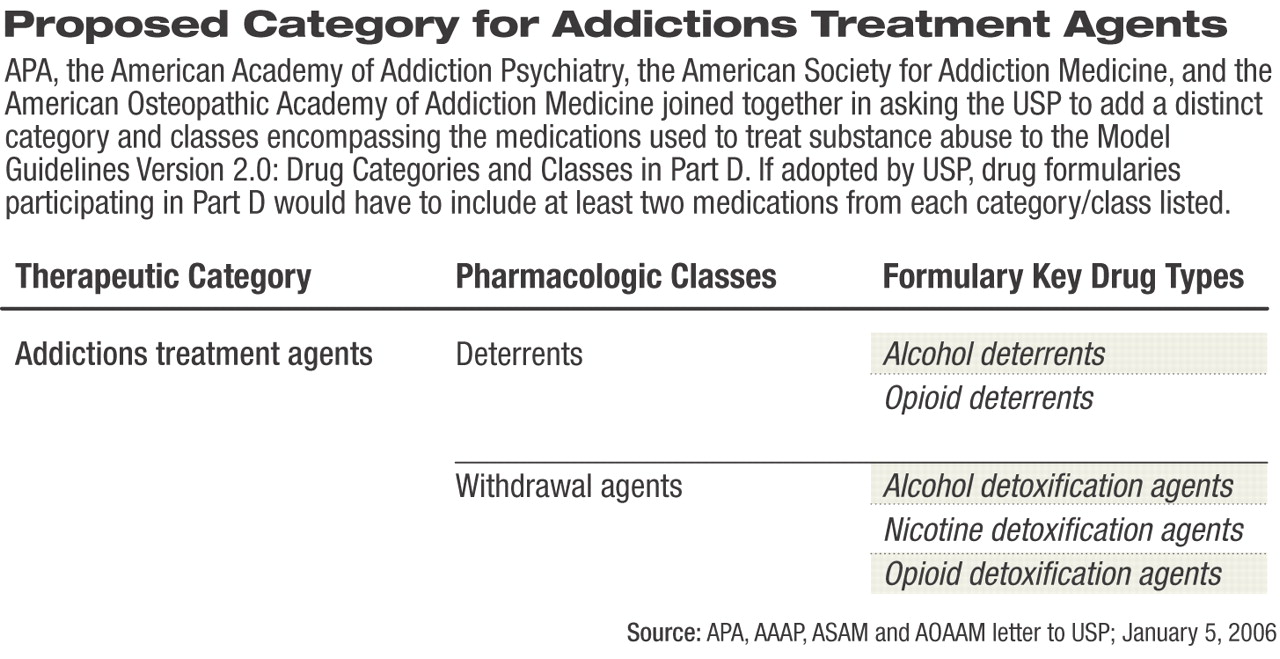
While these medications produce their clinical effects through different mechanisms of action, they can generally be divided into two main classes: medications used to treat withdrawal symptoms and medications used to deter future use of a substance of abuse. Based on that guiding principle, APA and the other organizations strongly recommended that a new therapeutic category of “Addictions Treatment Agents” be added to the revised guidelines (see table at left).
Within the new category, drugs would be subdivided into a pharmacologic class of “Deterrents,” which would include key drug types for medications used to deter alcohol or opioid use.
The second drug class, labeled “Withdrawal Agents,” would contain three key drug types—medications used as alcohol detoxification, nicotine detoxification, and opioid detoxification.
Some addiction treatment medications are listed in the USP revision for 2007. However, they are lumped into the broad category of “Antidotes, Deterrents, and Toxicologic Agents” along with “Ion Exchange Resins” and antivenins. This listing “seems an inappropriate categorization,” the presidents of the four organizations wrote. Substance use disorders, they continued, are highly prevalent medical conditions with significant morbidity and mortality. “Given the proven effectiveness and evidence base of these pharmacologic treatments, our organizations strongly believe that these medications should be included in the revised guidelines.” ▪