From 2003 to 2005, the U.S. Food and Drug Administration (FDA) released a series of public-health advisories and required a black-box warning be added to the labeling information of antidepressants regarding increased risk of suicidality in pediatric patients. These much publicized alerts, aimed primarily at pediatric depression treatment, appear to have led to a spillover effect on the diagnosis and treatment of adults with depression, according to a study in the August American Journal of Psychiatry.
The various public health advisories and alerts since October 2003 issued by the FDA contained broad warnings and admitted a lack of conclusive evidence on the suicide risks in adult patients using antidepressants. This past May the black-box warning was expanded to young adults aged 19 to 24 and wording pointing out the benefits of antidepressants was added (see “
FDA Timeline on Issuing Warnings”). These changes occurred after the time frame of the current study.
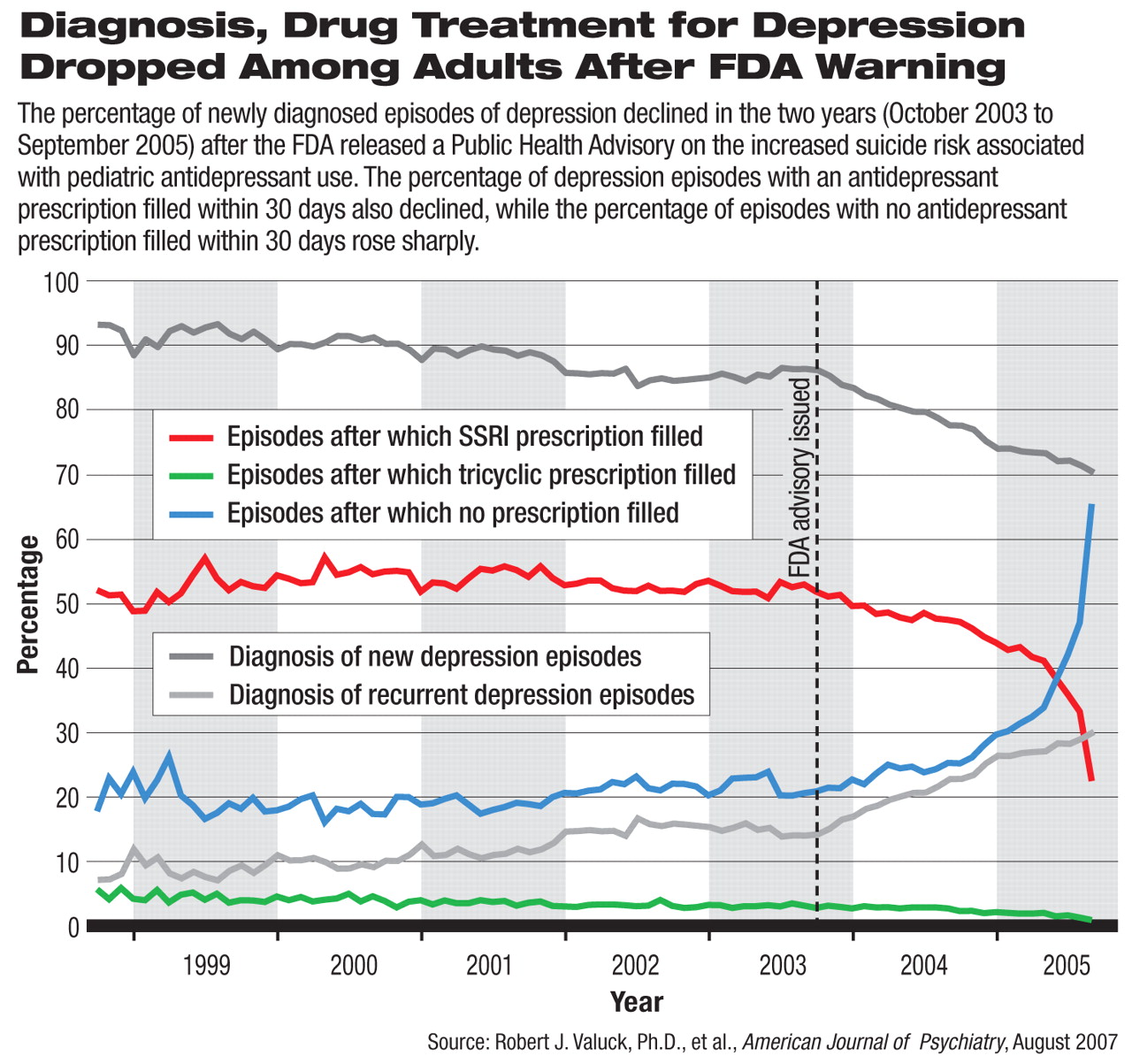
The rate of diagnosis of new depressive episodes saw a decreasing trend in the two years after October 2003, when the FDA issued its initial public health advisory on antidepressant risks. The percentage of antidepressant prescriptions filled after diagnosed episodes also declined significantly during the same period, suggesting undertreatment or lack of treatment for adult depression.
The study examined medical and pharmacy claims data from a nationwide database that included 47 million people who were covered in 85 managed-care plans. Based on the ICD-9-CM codes for the visits and on pharmacy data, more than 475,000 episodes of depression in about 400,000 adults (ages 19 to 89) were included in the analyses.
The researchers used monthly aggregate data and statistical regression to map the trends of depression diagnosis and antidepressant prescriptions from October 1998 to September 2005—two years after the initial FDA advisory.
New Diagnosis Rates Declined
Robert Valuck, Ph.D., an associate professor in the Department of Clinical Pharmacy at the University of Colorado at Denver, and colleagues found that the annual rates of diagnosed episodes of depression had been increasing steadily from 1999 to 2004, but dropped precipitously in 2005. If the increasing trend had continued, they predicted that the rate of depression diagnosis in 2005 would have been about 40 percent higher than the actual rate of diagnosis, a statistically significant difference.
Compared with the trend in the five years before the first FDA advisory, the number of new episodes of diagnosed depression dropped significantly faster in the two years after the advisory, accounting for a lower percentage of all depression episodes. In this study, new episodes of depression were defined as a diagnostic claim of depression for someone who had not had a depression diagnosis in the prior four months or any antidepressant prescription claims in the prior three months.
The authors examined the practice type of physicians who diagnosed depression. Before the advisory, nearly half of the depressive episodes were diagnosed by primary care physicians, and this share was growing at an annual rate of 5.4 percent. After the advisory, the absolute proportion of depressive episodes diagnosed by primary care physicians was more than half of all diagnosed episodes, but the trend was in fact reversed, declining at an annual rate of 2.86 percent, a statistically significant change. Thus, the FDA warning may have made primary care physicians more reluctant to diagnose depressive episodes.
The pattern of depression diagnosis among psychiatrists and mental health professionals did not significantly change after the advisory.
Fewer Patients Get Antidepressants
The authors also analyzed antidepressant prescription claims that were filled within 30 days of each diagnosed depression episode. In the five years before the FDA advisory in October 2003, an average of 53 percent of depressive episodes were accompanied by pharmacy claims for selective serotonin reuptake inhibitors (SSRIs), with a slightly growing trend over time. The percentage of episodes in which an SSRI prescription was filled declined sharply after the advisory, with an annualized decreasing rate of 13.15 percent. By September 2005, less than a quarter of all depressive episodes were accompanied by a filled prescription for an SSRI.
Tricyclic antidepressant prescriptions saw a milder decline. No other antidepressants saw significant increases. The percentage of depressive episodes with no accompanying antidepressant prescriptions filled grew at an annualized rate of 20.62 percent between October 2003 and September 2005.
The increasing number of patients not receiving antidepressant drug therapy did not appear to be getting other treatment, either. The researchers found no statistically significant change in the percentage of depressive episodes that were followed by at least one psychotherapy visit within 180 days. Similarly, no change was seen after the advisory in atypical antipsychotic or anxiolytic prescriptions within 30 days after depression diagnosis.
“The most alarming element in our findings was that rates of diagnosis of depression went down after the warnings came out,” said Valuck. “It was not unexpected that rates of antidepressant prescribing would go down and that there could be compensatory increases in the use of other psychotropic medications and/or psychotherapy (although we didn't see any). But it was not expected that diagnosis rates would fall.”
Similar decreasing trends in the diagnosis and treatment of depression in children and adolescents were detected in a study using the same managed-care-claims database and published in the June American Journal of Psychiatry (Psychiatric News, June 15).
The study “Spillover Effects on Treatment of Adult Depression in Primary Care After FDA Advisory on Risk of Pediatric Suicidality With SSRIs” is posted at<ajp.psychiatryonline.org/cgi/content/full/164/8/1198>.▪