Approved by the U.S. Food and Drug Administration for promoting wakefulness in patients with narcolepsy, sleep apnea, and shift-work sleep disorder, modafinil (Provigil) is being tested in clinical trials for a variety of other indications ranging from unipolar depression to multiple sclerosis.
In a study published in the August American Journal of Psychiatry, modafinil showed favorable effectiveness as an addon drug to mood stabilizers, compared with placebo, in patients with bipolar depression.
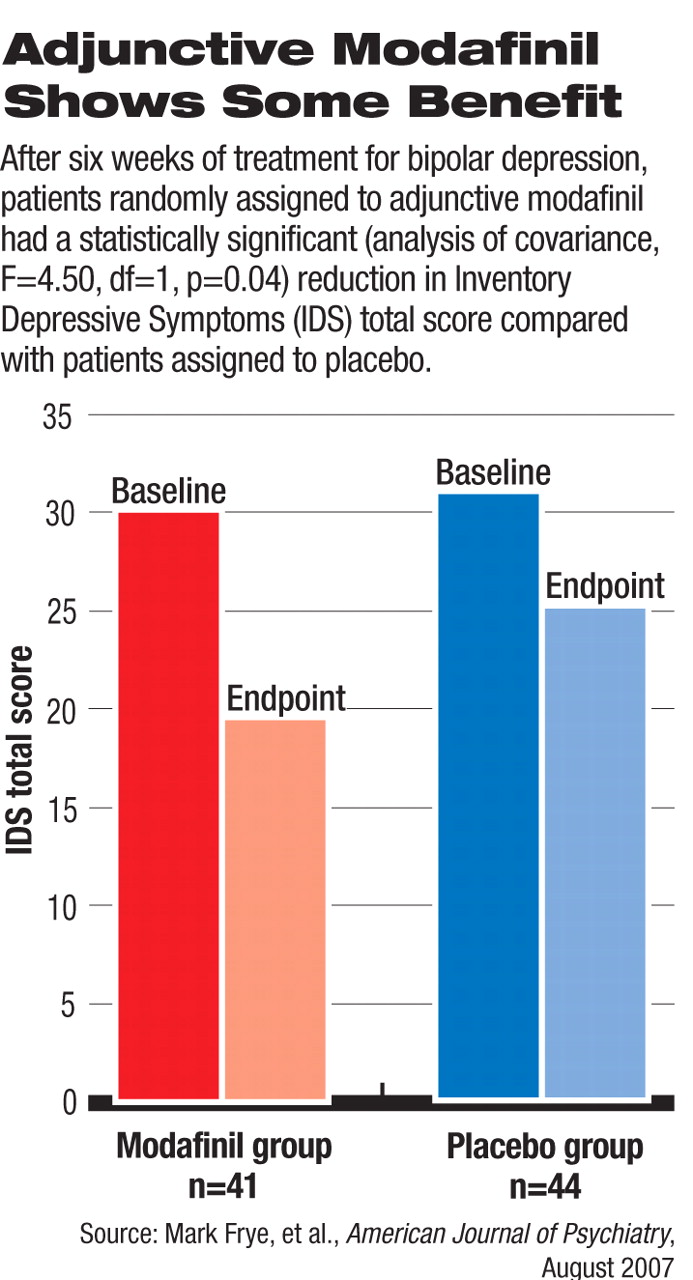
In this double-blind, placebo-controlled study, 85 patients were randomly assigned to either the modafinil (n=41) or placebo (n=44) group. The patients all had a diagnosis of bipolar disorder with depressive symptoms despite being on stable doses of mood stabilizers for at least two weeks before enrolling in the study. About half were also taking antidepressant drugs. The inclusion criteria required that patients have moderate depression, defined as a score of at least 16 on the Inventory of Depressive Symptoms–Clinician Rated (IDS) as an indication of inadequate response to their current treatment.
Patients were given one 100 mg tablet of modafinil or placebo daily for one week and titrated up to two tablets daily if necessary. Twelve and 15 patients dropped out of the modafinil and placebo groups, respectively, resulting in 58 patients completing the study.
At the end of six weeks, the reductions in the IDS score, four-item fatigue-and-energy subset of the IDS, and Clinical Global Impression–Bipolar Version scale (CGI-BP) depression severity item score in the modafinil group were significantly greater than the reductions in the placebo group. In the modafinil group, 44 percent of patients achieved a 50 percent or greater improvement in IDS score, significantly better than the placebo group, in which 23 percent achieved such response.
The remission rates (defined as an IDS score of less than 12 at the end of the study) were 39 percent and 18 percent in the modafinil and placebo groups, respectively, also statistically significant.
During the study period, six patients in the modafinil group and five in the placebo group experienced hypomania or mania, including one in each group who needed to be hospitalized for mania.
This study was funded by the Stanley Medical Research Institute, a supporting research organization under the Treatment Advocacy Center, which is a nonprofit organization based in Chevy Chase, Md. Cephalon Inc., the manufacturer of modafinil, provided the active drug and placebo and a grant for patient recruitment and advertisement. The researchers are affiliated with the departments of psychiatry at the University of California, Los Angeles; University of Texas Southwestern Medical Center; and Ludwig-Maximilians University, Munich, Germany. The study was conducted at these three academic centers.
In an accompanying editorial, R.H. Belmaker, M.D., of Ben-Gurion University of the Negev, Beer-Sheba Mental Health Center in Israel, said that “it is biologically plausible that modafinil might be useful in some cases of bipolar depression.” He cautioned about the similar rates of mania-switching found in the modafinil and placebo groups, as the modafinil dose in the study was relatively low (mean dose 177 mg/day). The recommended dosage of modafinil for treating sleep disorders is 200 mg, according to the product labeling information.