Serious injuries, disabilities, and deaths related to the use of prescribed medications nearly tripled in recent years, according to research based on Food and Drug Administration (FDA) data.
The total annual incidents of these events rose from 34,966 in 1998 to 89,842 in 2005, a 2.6-fold increase, according to a study in the September 10 Archives of Internal Medicine. A total of 467,809 such incidents were reported over that eight-year period to the FDA's Adverse Event Reporting System (AERS). The AERS is described as the world's largest database of voluntary spontaneous reports of adverse drug reactions and medication errors for prescription drugs, biological products (except vaccines), and over-the-counter drugs. The voluntary reports are submitted directly to the FDA by medical professionals and consumers through MedWatch or to drugmakers, which must forward them to the FDA.
The researchers determined that the rise in injuries and deaths was attributed to “a minority of important drugs.” Drugs most associated with injuries and deaths were disproportionately painkillers and immune-system modifiers. Just 298 approved medications (20 percent) accounted for 87 percent, or 407,394, of the injuries and deaths reported.
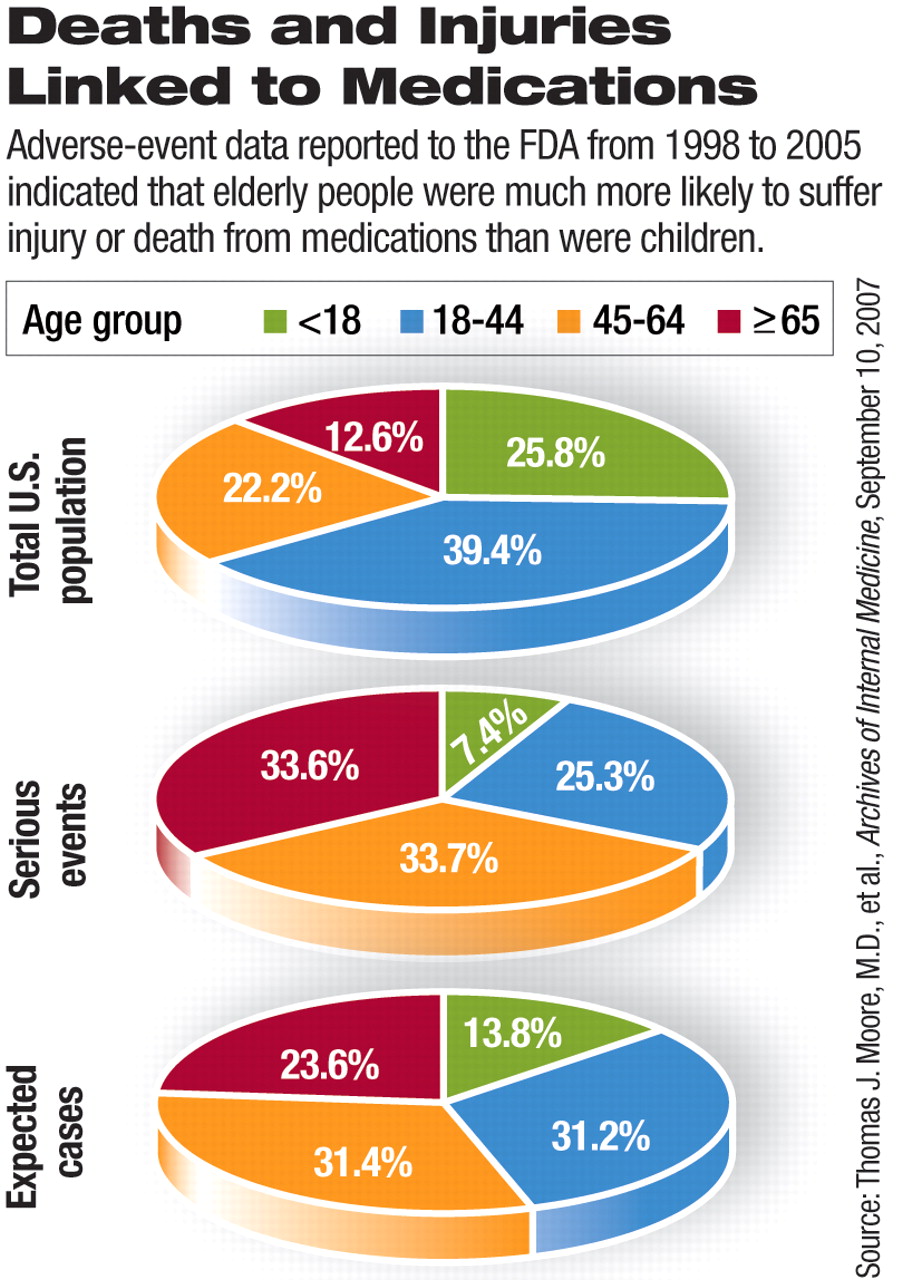
The adverse impact of medications was disproportionate among adults and children. A proportionately higher number of elderly patients were injured or died from medications, while proportionately fewer children were affected. Although researchers expected 13.8 percent of children under age 18 to have serious complications, based on the number of prescriptions written for this population group, only 7.4 percent of reports of serious adverse events involved children.
People aged 65 and older accounted for 33.6 percent of the serious adverse events reported, although the number of prescriptions written for this age group led researchers to expect they would account for only 23.6 percent.
The causes behind the increase were varied, according to the study authors. Twenty-five percent of the rise in adverse events, they estimated, was likely due to population growth and more intense use of medication therapy. Another 15 percent of the increase was attributed to 13 “biotechnological products,” including immuno-modulators created through genetic engineering, which were new to the market in the study period. The number of serious adverse events associated with 13 prominent biotechnology products grew nearly 16-fold, from 580 in 1998 to 9,181 in 2005.
Contrary to the researchers' expectations, a modest and declining share of serious adverse events were linked to drugs that were the subject of safety withdrawals. Drugs linked to safety withdrawals accounted for less than 10 percent of serious adverse events reported and declined from 26.4 percent of health incidents in 1999 to less than 1 percent in 2005.
Alfred Herzog, M.D., chair of APA's Committee on Patient Safety, said that the jump in reported adverse impacts of medication was probably at least partially linked to stepped-up reporting requirements that many states implemented in the early part of this decade. However, the ongoing shortage of nurses and hospital beds may have led to revisions of administrative medication guidelines that are more expedient but offer less protection to patients.
Among psychiatric medications, the antipsychotic clozapine was the most frequent drug suspected in death and serious nonfatal outcomes, with 3,277 such cases reported over the eight years. The next highest ranked was the antipsychotic risperidone, which was the suspect drug in 1,093 deaths or serious injuries during the study period.
The study excluded any adverse events reported as part of FDA drug trials.“ Health professionals” provided 70 percent of all adverse event reports, including 82 percent of all reported deaths.
Future efforts to improve drug safety, wrote the study authors, will require “more accurate and capable systems to monitor postmarketing” injury and death. The growing toll shows that the existing system is inadequate to protect patients and underscores the need for legislative, institutional, and policy changes.
The increase of adverse health events underscores the urgency for Congress to require electronic prescribing in Medicare, according to the Pharmaceutical Care Management Association (PCMA).
“This study found that seniors are disproportionately affected by serious adverse drug events, which underscores why we need to require e-prescribing in Medicare immediately. Requiring e-prescribing in Medicare could prevent up to 1.9 million medication errors over the next decade,” said Mark Merritt, president and CEO of PCMA, in a statement
E-prescribing technology aims to avoid adverse drug events by providing physicians with a comprehensive patient medication history and automatic electronic safety alerts before the prescription is sent to the pharmacy. A recent study by the Gorman Health Group also concluded that requiring e-prescribing in Medicare could prevent up to 1.9 million medication errors over the next decade.