The public health would benefit significantly if the decision makers who determine coverage limitations in private and public insurance plans listened to, and learned from, the major take-home messages produced by recent studies funded by the National Institute of Mental Health (NIMH), says the lead researcher on one of those clinical trials.
He is John March, M.D., M.P.H., a professor of psychiatry and chief of child and adolescent psychiatry at Duke University Medical Center. He heads up the Treatment of Adolescents With Depression Study (TADS) on which of series of reports appeared in the December 2006 Journal of the American Academy of Child and Adolescent Psychiatry (JAACAP).
In the TADS, March and his colleagues showed that across numerous outcome domains, the combination of the antidepressant medication fluoxetine and cognitive-behavioral therapy (CBT) yielded the most effective weapon against adolescent depression, compared with medication alone, CBT alone, or placebo.
Most importantly, March said, the TADS team documented a direct effect particular to pairing the SSRI fluoxetine with CBT (COMB)—a near complete elimination of suicidal thoughts and behaviors in the depressed teens.
“Depending upon whose numbers you cite, there are about 2 million kids each year in the U.S. who end up getting medical attention because of a suicide attempt,” March noted. “Conservatively, if even half of those received COMB treatment, and then only half of those who received COMB saw a significant reduction or complete alleviation of their suicidality, think about the number of lives that would be positively impacted.”
In the JAACAP report series, March and his fellow TADS investigators detailed the secondary outcomes of the TADS. Without exception, the data confirmed researchers' initial reports that COMB was more effective across numerous secondary domains at treating adolescent depression than fluoxetine alone, CBT alone, or clinical management plus a placebo pill.
“If you want the treatment that gives you the best chance of benefit and the smallest risk, it's definitely the COMB treatment. It doesn't matter what [outcome variable] you look at,” March told Psychiatric News. “COMB is better across multiple domains: better [at promoting] remission, better [at improving] patient functionality, better in [maintaining or improving] quality of life, better [as measured] by adolescent self-report. And COMB is associated with fewer side effects.”
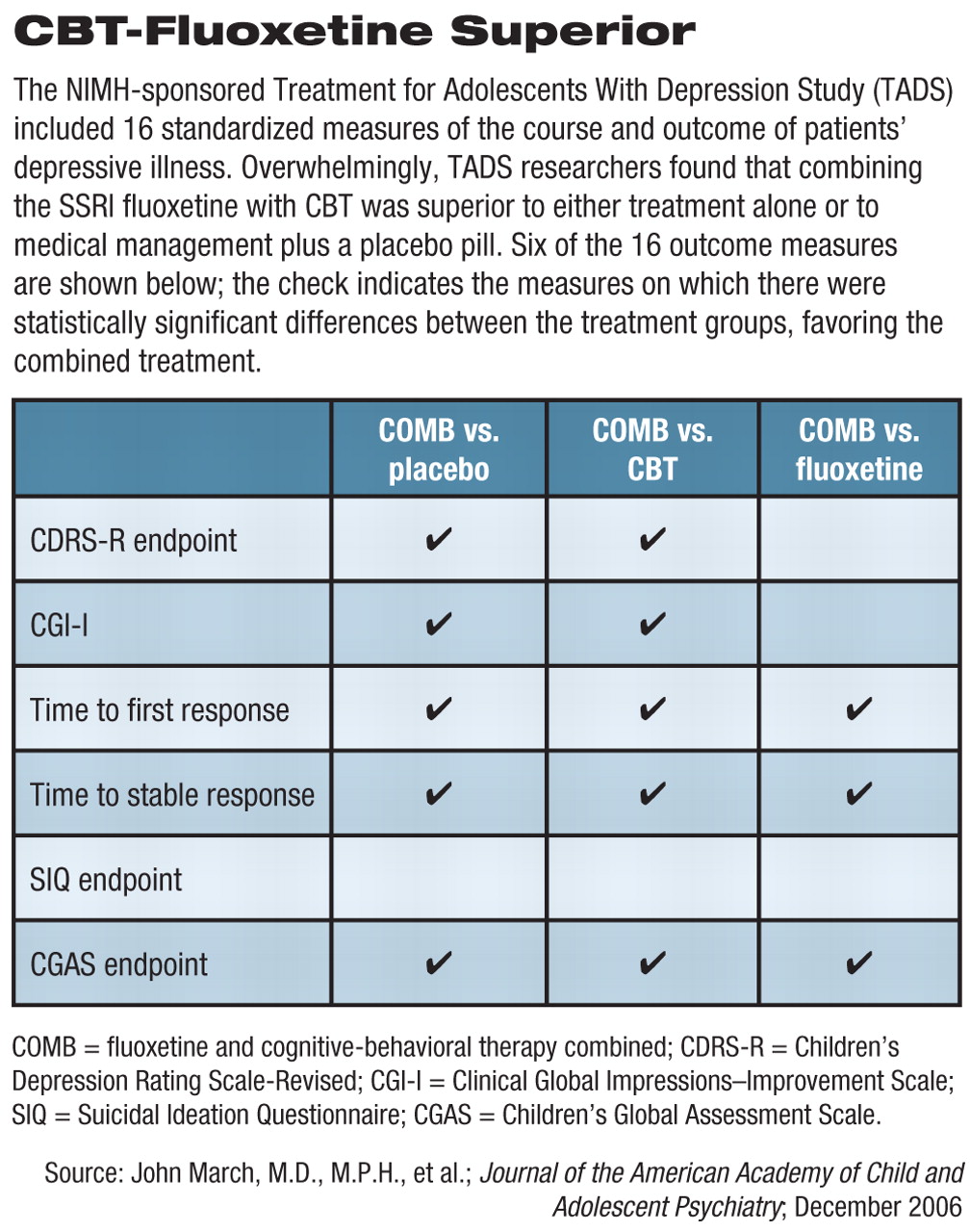
Study Outlined
The TADS protocol involved researchers and clinicians who treated 439 adolescents with major depression at 13 academic centers across the country. The goal was to evaluate the effectiveness in a real-world setting of CBT alone, fluoxetine alone, and the combination of CBT and fluoxetine and then compare them with placebo.
The CBT arm of the study involved a “skills-oriented treatment based on the assumption that depression is either caused by or maintained by depressive thought patterns and a lack of active, positively reinforcing behavior patterns.”
In the first phase of the study, which ran 12 weeks, the patients in the CBT group completed 15 50- to 60-minute sessions. The patients in the fluoxetine and placebo groups were monitored in six 20- to 30-minute sessions.
Two primary outcome measures were used: the patients' change in score on the Children's Depression Rating Scale-Revised (CDRS-R) at weeks 6 and 12, compared with baseline; and the 12-week rating on the Clinical Global Impression–Improvement (CGI) scale. A CGI score of 1 (much improved) or 2 (very much improved) was defined as a “response” to treatment.
The overall comparative effectiveness of the four treatments was reported in 2004 (Psychiatric News, September 3, 2004). Briefly, all 439 patients improved from baseline through week 6 and from week 6 through week 12. At week 12, 71 percent of patients in the COMB group were rated as responders, compared with 60.6 percent in the fluoxetine group, 43.3 percent in the CBT group, and 34.8 percent in the placebo group. The superiority of the COMB and fluoxetine treatments, compared with the CBT and placebo groups, was statistically significant. A report on outcomes at 36 weeks is currently under review for publication.
As reported in the December JAACAP series, secondary outcome measures studied at the 12-week mark included comparative rates of remission (as opposed to the previously reported rates of response), speed of response, function and quality of life, predictors and/or moderators of outcome, and safety. Overall, 16 clinical outcome measures were compared between the four groups.
The COMB treatment proved to be superior to placebo on 15 of the 16 secondary outcome measures, to CBT on 14 of 16, and to fluoxetine on 8 out of the 16 measures (see table).
Fluoxetine proved to be superior to CBT on eight of the 16 measures and to placebo on 7 of the 16 measures. CBT did not differ from placebo on any of the secondary outcome measures.
Findings Shed Light on Suicidality Issue
The TADS protocol was finalized well before the reemergence of concern in 2003 over the possibility of an increased risk of suicidality associated with antidepressant medications (see
page 1). However, TADS researchers used several secondary measures to assess baseline suicidality and to monitor for potential changes in suicidality over time within the four study groups, including the potential for treatment-emergent suicidality possibly associated with fluoxetine. Suicidality was assessed using the Suicidal Ideation Questionnaire (SIQ), the suicide item on the Children's Depression Rating Scale–Revised (CDRS-R), and the Clinical Global Impression–Improvement scale.
At baseline, nearly 30 percent of TADS patients were found to have clinically significant suicidality, and 2 percent were rated as“ extremely suicidal.”
Even though suicidality improved markedly across all of the treatment conditions, 24 clinician-identified suicide-related events occurred over the course of the 12-week study. Suicide-related events occurred more frequently in the fluoxetine group (10 patients with events, or 9.2 percent of the group), compared with the COMB group (5 patients, 4.7 percent) and the CBT group (5 patients, 4.5 percent). The placebo group had the lowest number of events (3 patients, 2.7 percent).
While no suicides occurred, there were five suicide attempts (two in the COMB group, two in the fluoxetine group, and one in the CBT group).
“One of the most striking results out of TADS,” March told Psychiatric News, “is the fact that a patient's assignment to the COMB treatment group [significantly reduces] the small risk that you have for a suicidal event when taking fluoxetine alone. That suggests that CBT in TADS had a direct effect of mitigating suicidality—it demonstrated a protective effect.”
Public Health Is Bottom Line
“From a public health point of view,” March continued,“ it would be a tremendous benefit if the take-home messages from TADS were operationalized by [both large private and public insurers].”
First, he pointed out, “the COMB treatment is clearly better than either monotherapy alone; second, the COMB treatment [appears to significantly reduce] the risk of suicidality you might encounter if you take medication alone; and third, the TADS findings are robust enough that they probably should always be mentioned during informed consent when a patient is considering treatment options.”
Access to mental health services in the United States, March continued, is woefully inadequate and not based on the most recent scientific findings. With other medical disorders, he explained, multi-modal treatment is not discouraged, but rather highly encouraged.
“You would never today be able to tell orthopedists or rheumatologists that they can't [prescribe] physical therapy for their arthritis patients in addition to taking medications,” March said.“ You would never be able to tell a diabetic patient that there is no such thing as nutritional counseling or diet and exercise programs to supplement their medication.”
Why, March asked, does the health care system in the U.S. today still allow limits on coverage so that many patients have access only to antidepressant medications, with little if any possibility of accessing other proven treatment tools, including proven psychotherapy techniques and other psychosocial interventions?
“It simply makes absolutely no sense at all,” March concluded,“ CBT is the physical therapy of mental health care.”
The potential benefit to the public health of using COMB treatment for adolescents is enormous, March said. If combination treatment—with both antidepressant medications and psychotherapy—were the standard of care,“ we would almost certainly see improvements in multiple outcomes, including a direct reduction in suicidality among depressed adolescents.”
JAACAP's special section on TADS can be accessed online at<www.jaacap.com/pt/re/jaacap/toc.00004583-200612000-00000.htm>.▪