Over half of adolescents who don't respond to a first antidepressant improve when switched to a combination of a different antidepressant and cognitive-behavioral therapy (CBT). This is a key finding of an NIMH-funded clinical trial, the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA).
The combined therapy also produced a greater response than placing patients on another medication alone, suggesting that persistence may pay off in this population, said the authors in the February 27 Journal of the American Medical Association.
Previous trials have shown that SSRI antidepressants or CBT or the combination can produce an adequate clinical response in up to 60 percent of treated adolescents, the authors noted, but, “There are no empirical studies to guide clinicians regarding the management of adolescents with depression not responsive to an initial treatment with an SSRI.”
Previously, the Treatment of Adolescent Depression Study (TADS) demonstrated a similar benefit for combined therapy, but it tested a treatment-naïve group of depressed adolescents. TORDIA differed in two ways from TADS. It was aimed at youth who had not responded to a first round of treatment, and it included more chronically depressed adolescents (averaging two years) and more with suicidal ideation (59 percent)—groups often excluded from clinical trials, said lead author David Brent, M.D., in an interview with Psychiatric News.
“This was a much more difficult-to-treat sample,” said Brent, a professor of psychiatry at the University of Pittsburgh School of Medicine and Western Psychiatric Institute and Clinic. “We came to see that it would be unethical not to study people at high risk.”
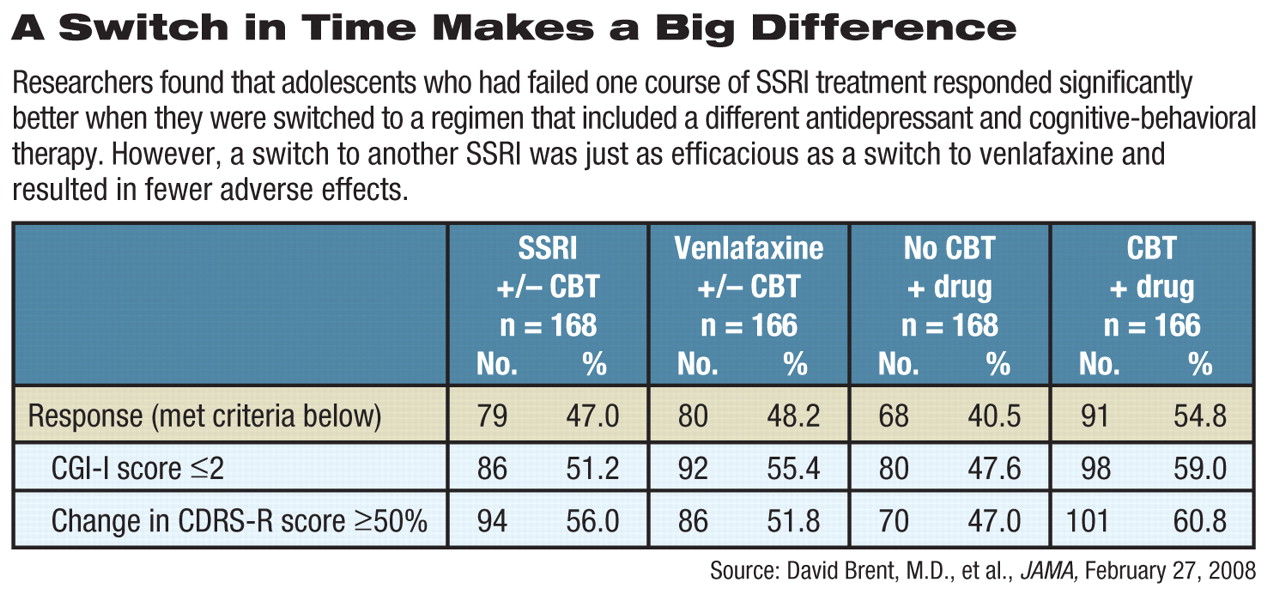
The TORDIA study recruited young people, aged 12 to 18, who had not responded to at least an eight-week regimen of an SSRI antidepressant and who were not receiving CBT. In all, 334 patients at six institutions were randomized to one of four treatment options: a new SSRI alone (fluoxetine, paroxetine, or citalopram); an SSRI along with CBT; venlaflaxine with CBT; and venlaflaxine alone. Venlaflaxine, an SSRI/SNRI, was included because it has shown value in some trials in adults, said the authors.
The FDA's black-box warning, issued in the midst of the trial, may have cut into recruitment efforts, but may also have prompted improved procedures for monitoring any emergence of suicidal ideation or behavior and helped to fine-tune rescue protocols for intervention if signs of suicidality appeared, said Brent.
About 86 percent of the participants completed 12 weeks of the treatment protocol. Those who left did so because of adverse effects, nonadherence or withdrawal of consent, or need for out-of-protocol treatments. Participants were mostly white (82 percent), female (70 percent), and middle class ($61,000 annual median family income). However, compared with other trials, such as TADS, participants showed higher rates of two risk factors—chronicity and suicidal ideation—that made this sample more like the patients seen in routine community practice.
CBT Makes the Difference
About 55 percent (91 of 166) of patients treated with CBT and an antidepressant evinced an adequate clinical response, compared with those treated with an SSRI (47 percent, 79 of 168) or venlafaxine (48 percent, 80 of 166) but without CBT.
Patients were evaluated with the Clinical Global Impressions-Improvement Subscale and the Children's Depression Rating Scale-Revised.
The trial results extend TADS to “a more chronically depressed, treatment-resistant population,” the authors said.
Results with CBT were achieved with a median of nine sessions, leading the authors to speculate that more sessions might produce a larger effect.
When planning the study, the researchers thought that switching to venlafaxine would prove superior to use of another SSRI, but that hypothesis was not borne out by TORDIA's findings. Treatment effects were similar to those for SSRIs, but side effects were slightly more frequent.
Regarding the lack of superiority for venlafaxine, Brent said, “I would suggest avoiding it as a second step although it might be useful as a third step.”
Sleep Problems Linked to Response
One unexpected finding from TORDIA was that patients who used sleep medications had a poor response to treatment.
“We don't know if this was due to the hypnotic drugs themselves, or to drug-drug interactions, or to the underlying sleep problems,” said Brent. “But it's important to pay attention to sleep difficulties when treating refractory depression.”
There was no difference between the treatment arms in the proportion of harm-related events (suicidal ideation, suicide attempt, or self-injurious behavior). There were 18 suicide attempts, but no completed suicides among the participants despite a greater incidence of suicidality at intake than is usual in most clinical trials, said Brent.
The authors also noted that the response to CBT varied from site to site despite consistent training and monitoring of therapists. Those variations were due to baseline variables among study participants (such as hopelessness) that predict outcomes, said Brent. Adjusting for those differences eliminated the variations. Anyone planning future multisite trials should understand that such variability is inevitable and will have to be factored in when calculating sample size to achieve the power needed in the trial, he said.
In the end, clinicians can offer some hope to adolescents with depression who have not responded to a first course of antidepressants, the researchers said. “[D]espite a first unsuccessful treatment for depression, persistence with additional appropriate interventions can result in substantial clinical improvement.”
Brent and his coauthors are continuing to follow the trial participants.“ We're working now on delineating the response at six months and have followed these patients out to 72 weeks,” he said. “But I predict that the data will be difficult to interpret. A lot of things can happen over that time, and what happens during the initial treatment becomes less and less relevant.”