Older adults with dementia experience significant weight gain and worsening cholesterol levels when they are treated with second-generation antipsychotic (SGA) medications in a pattern similar to younger patients taking antipsychotics for schizophrenia or bipolar disorder, according to new analyses of data from the Clinical Antipsychotic Trials of Intervention Effectiveness–Alzheimer's Disease (CATIE-AD).
The CATIE-AD study, a large, randomized, double-blind, placebo-controlled trial funded by the National Institute of Mental Health, was conducted between April 2001 and November 2004 to investigate the effectiveness of SGAs in treating psychosis and agitation in patients with Alzheimer's disease (AD).
The study investigators had previously reported that the primary outcome, time to discontinuation of the study drug for any reason in 36 weeks of treatment, did not differ significantly among patients treated with olanzapine, quetiapine, risperidone, and placebo (Psychiatric News, November 3, 2006). Despite modest effectiveness in some of the clinical indicators with active treatments, many patients discontinued antipsychotics because of intolerable adverse effects.
The current study, by Ling Zheng, M.B.B.S., Ph.D., and colleagues, focused on analyzing the weight and metabolic effects of SGAs in study patients. It was published online in AJP in Advance on April 15.
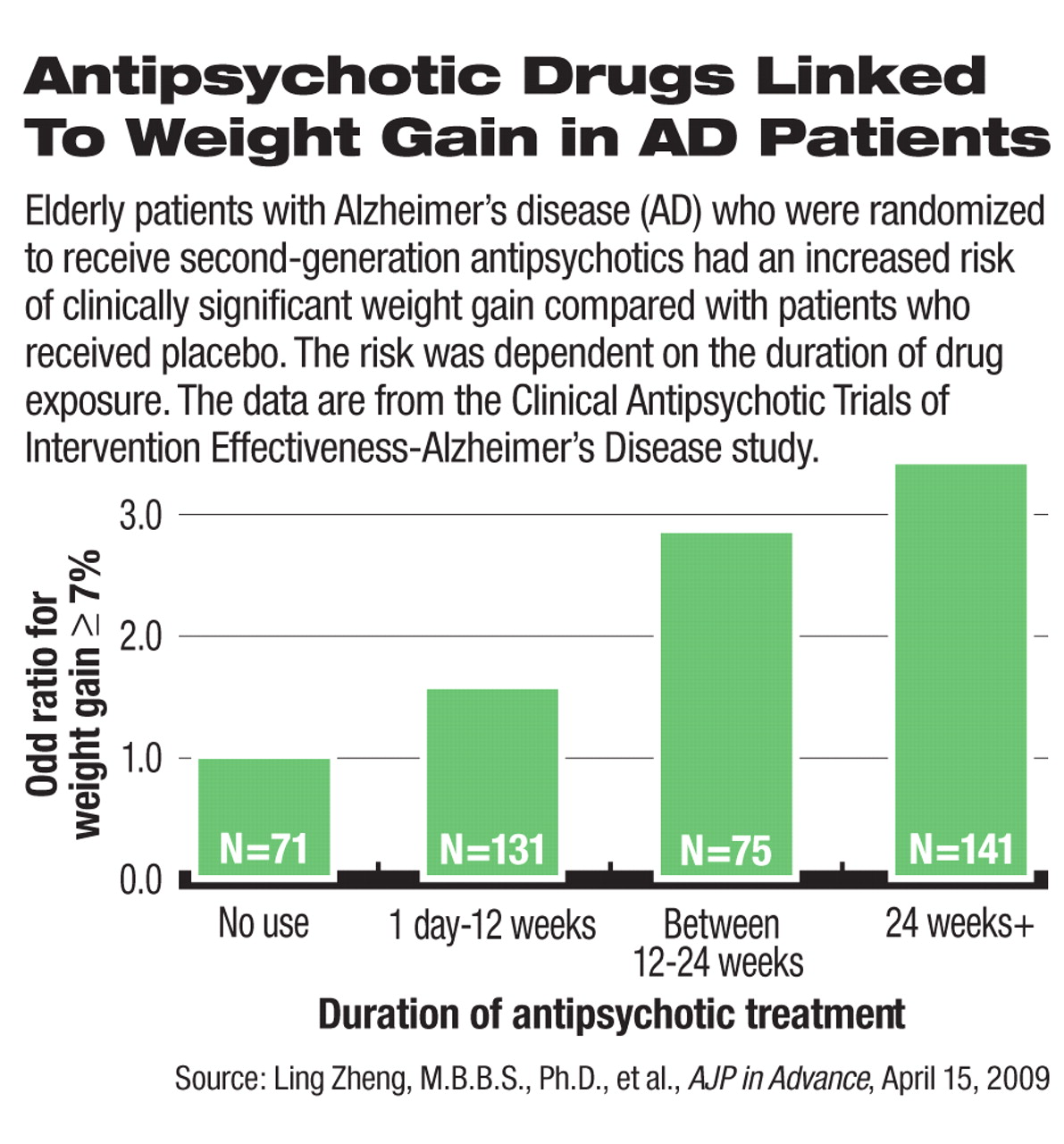
Among the ambulatory Alzheimer's patients who participated in the CATIE-AD trial, female patients, but not male patients, had statistically significant weight gain and an increase in body mass index (BMI) from baseline. The authors found a significant association between the duration of SGA exposure and weight gain: The longer that patients took SGAs, the more likely they would gain at least 7 percent of their body weight, a conventional standard for clinically significant weight gain. On average, female patients gained 0.14 pounds per week while taking SGAs. In subgroup analyses, the weight and BMI increases reached statistical significance in patients who were randomly assigned to olanzapine or quetiapine.
In addition to weight gain, high-density lipoprotein (HDL) cholesterol level decreased and waist circumference increased significantly in patients on olanzapine compared with those on placebo. Increased waist circumference and decreased HDL cholesterol are both associated with increased risk of cardiovascular diseases. The analyses did not show a significant effect on blood pressure, triglyceride, and glucose levels associated with SGA use.
Unfavorable metabolic effects associated with SGAs, including weight gain, elevated cholesterol and triglyceride levels, and altered glucose regulation/insulin insensitivity, mostly reported in child and adolescent patients taking these medications, have caused much clinical concern and media attention in recent years. The metabolic effects in older adults have been less studied. The average age of patients in the CATIE-AD study was 78 years.
“The important message from the study is that elderly patients with dementia receiving antipsychotics experience some of the same changes of metabolic syndrome that younger patients have,” said Lon Schneider, M.D., a professor of psychiatry, neurology, and gerontology at the University of Southern California Keck School of Medicine, in an interview.“ Whereas it took a while for the field to recognize these metabolic changes associated with antipsychotics in younger patients, it has taken longer for us to realize they occur in elderly patients as well.”
He continued, “The weight gain and metabolic effects were seen as early as 12 weeks into treatment and continued with longer use.” He noted that past clinical trials of SGAs, many sponsored by pharmaceutical companies, often lasted only six to eight weeks and did not adequately monitor metabolic effects.
None of the SGAs is approved by the FDA for treating symptoms of dementia. Despite the lack of evidence for efficacy from studies such as CATIE-AD, antipsychotics are often prescribed for dementia-related behavioral disturbances for which there is no approved drug therapy. A standard black-box warning is currently required by the FDA in the labels of all antipsychotics that warns “elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death” based on data from clinical trials.
The mounting evidence of potential harm, “in the context of these drugs not working very well,” should give clinicians pause for prescribing antipsychotics to dementia patients, Schneider suggested.
“Metabolic Changes Associated With Second-Generation Antipsychotic Use in Alzheimer's Disease Patients: The CATIE-AD Study” is posted at<ajp.psychiatryonline.org/cgi/reprint/appi.ajp.2008.08081218v1>.▪