A vaccine that keeps cocaine out of the brain shows promise as a viable treatment for cocaine addiction, but researchers must overcome several technical hurdles to increase the vaccine's effectiveness, a 24-week, randomized, placebo-controlled study shows.
The vaccine was made from succinyl-norcocaine attached to a manmade fragment of a cholera toxin. Once injected, the vaccine provokes an immune response that causes the body to produce a circulating antibody that binds to cocaine in the bloodstream. Thus, the cocaine molecules absorbed after snorting or injecting are “picked up” and “held” by the antibody, so that they are prevented from entering the brain to produce a high.
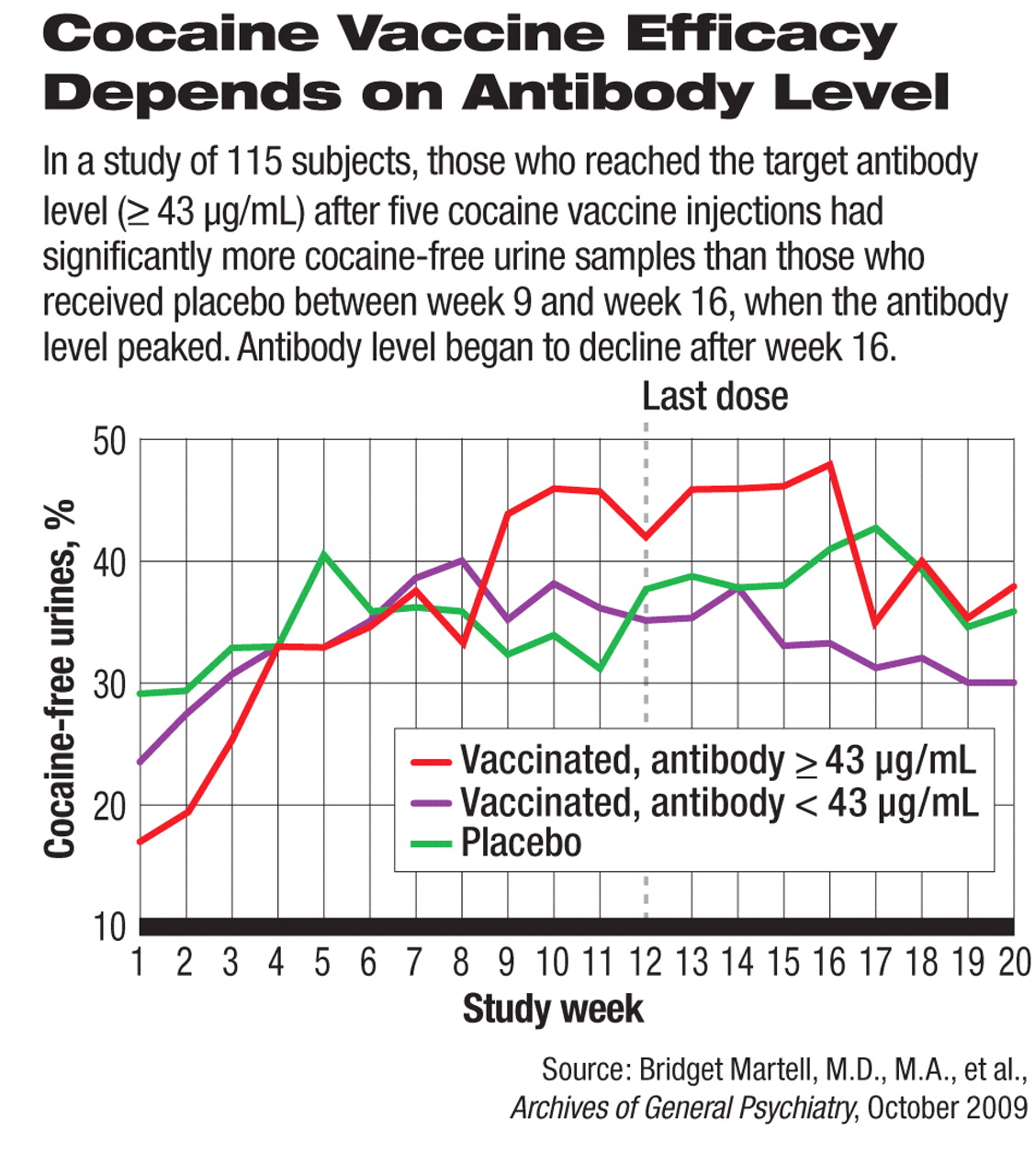
The study, conducted between October 2003 and April 2005, enrolled 115 patients at an outpatient methadone clinic who met the criteria for cocaine dependence. About half (58) were randomized to receive the vaccine injections at weeks 0, 2, 4, 8, and 12 (the first injection marked week 0). Fifty-five of the 58 subjects randomized to the vaccine treatment completed all five injections. Maximum anticocaine concentration was reached between week 12 and week 16. The rest of the subjects received placebo in a double-blind manner.
Twenty-one (38 percent) of the 55 vaccinated patients were able to generate a sufficient level of the endogenous anticocaine antibody at or above the target concentration of 43 μg/mL after five injections. These patients had a significantly higher rate of cocaine-free urine samples between week 9 and week 16 than patients who received placebo (see chart). Urine samples were collected and tested three times a week.
More than half (53 percent) of the patients with high antibody levels achieved a 50 percent reduction in cocaine use, significantly higher than the rate (23 percent) among the subjects whose antibody levels remained below 43 μg/mL. One of these patients produced no anticocaine antibody at all.
Once the patient achieves the targeted level, “[the antibody] really blocks cocaine's euphoric effect,” said Thomas Kosten, M.D., a professor of psychiatry and neuroscience at Baylor College of Medicine and the senior author of the study, at a press conference. “The participants with highest antibody levels had the greatest reduction of cocaine use, so there was a dose-response relationship.”
Currently there is no pharmacotherapy to treat cocaine addiction.
“Cocaine abuse is a serious public-health problem, as 1 out of 3 drug-related emergency room visits is associated with abuse of cocaine,” said Nora Volkow, M.D., director of the National Institute on Drug Abuse, at the press conference. The institute funded the study.
The anticocaine antibodies began to decline after week 16 in every vaccinated patient. A booster shot is sufficient to bring the antibody level back to the previous peak level, according to Kosten. Thus, he noted, clinical treatment with the vaccine will probably require a booster vaccination every two months to maintain a therapeutic antibody level.
The research goal now is to modify the vaccine to induce the cocaine-blocking level of antibody in more patients and to prolong the maintenance of the antibody, Kosten said. He and his colleagues have already developed some second-generation vaccines using different protein carriers and additive ingredients, he reported at the press conference. One of these vaccines is able to produce four to five times higher levels of antibody in animals and stay at the effective level for a longer period.
The researchers had chosen to study patients who were concurrently receiving methadone treatment to maximize study retention and prevent dropout. They also used contingency management strategies, such as paying a small sum for each completed visit, to encourage patients to stay in the study. Impressively, 55 of the 58 patients randomized to vaccination received 12 weeks of treatment, and 94 of all 115 participants completed the entire 24 weeks of the study.
Both Volkow and Kosten suggested that this vaccination strategy may be used to develop treatments for other substance abuse disorders. In fact, three nicotine vaccines are currently being developed by pharmaceutical companies. No company has yet shown an interest, however, in taking over the development of the cocaine vaccine, according to Kosten.