Tricyclic antidepressants may pose a higher risk of treatment-induced or worsening suicidal ideation than selective serotonin-reuptake inhibitors (SSRIs), but the effect appears to be limited to men, a large European study suggests.
The study, which compared one tricyclic and one SSRI, also demonstrates that both types of antidepressants reduce the overall level of suicidal thoughts over time in patients with moderate to severe depression.
The study was part of the Genome-Based Therapeutic Drugs for Depression (GENDEP) trial conducted in 10 countries in Europe and funded by the European Commission. The study sought to analyze how genetic factors influence the development of adverse effects and the efficacy of two types of antidepressants. Its goal also was to combine basic and clinical research to further the understanding of depression's biochemical mechanism and treatments.
In the study, published October 15 in the open-source, peer-reviewed online journal BMC Medicine, Nader Perroud, M.D., from the Institute of Psychiatry at King's College in London, and colleagues compared the incidence of new or worsening suicidal ideation after patients with major depressive disorder (MDD) began taking either nortriptyline or escitalopram.
About 800 patients aged 18 to 72 who were diagnosed with moderate to severe MDD were given either nortriptyline or escitalopram for 12 weeks in an open-label fashion. Over half of the patients were randomly assigned, while 41 percent with a history of adverse effects, nonresponse, or other contraindications to one of the antidepressants were assigned to the other drug.
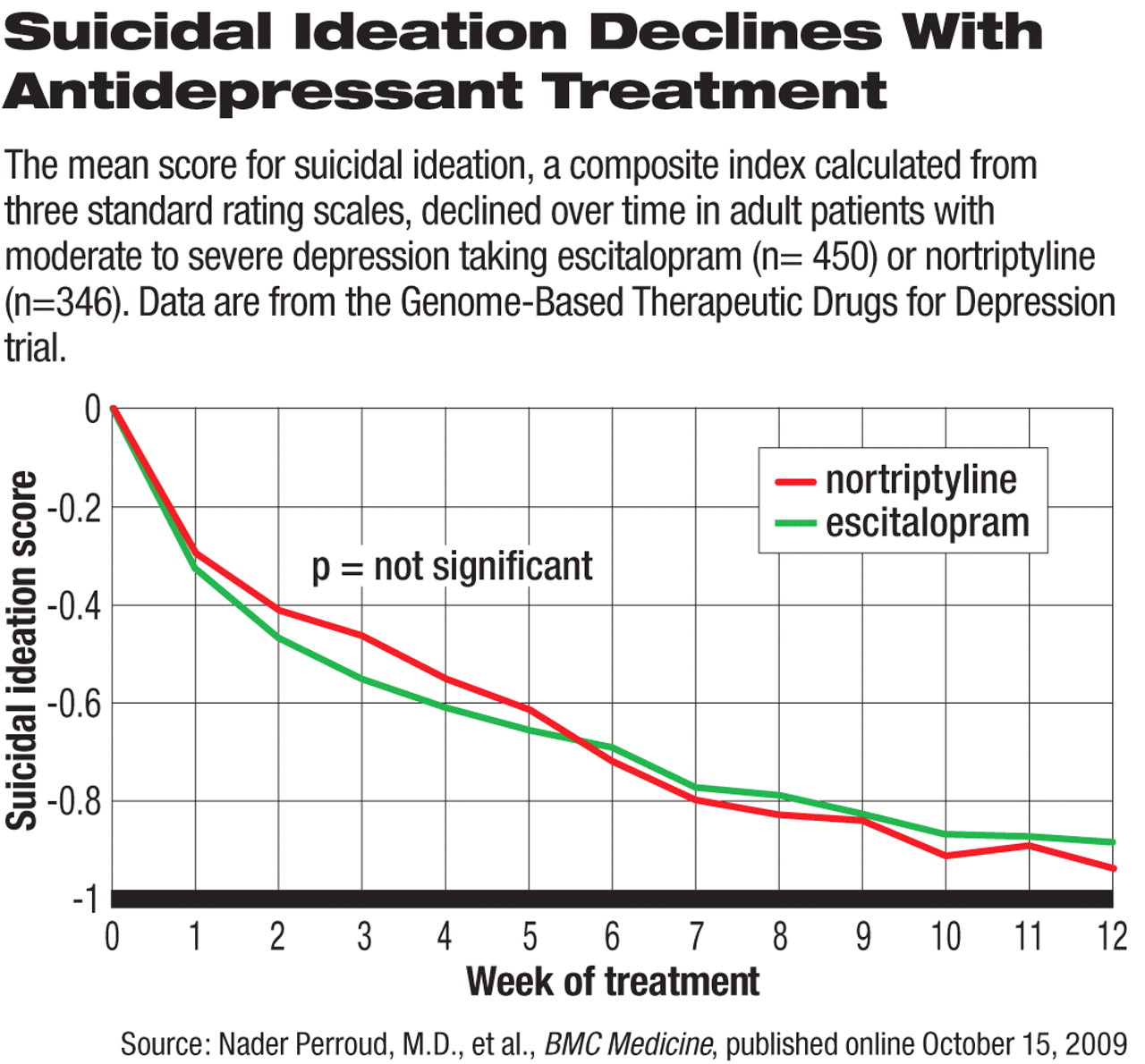
Suicidal ideation was measured with a composite score on the suicide-related questions in the Hamilton Rating Scale for Depression, Beck Depression Inventory, and Montgomery-Asberg Depression Rating Scale. The threshold for significant suicidal ideation was set on the basis of statistical analysis to capture significant suicidal ideation on at least two of the scales.
The primary endpoints were treatment-emergent suicidal ideation (TESI), defined as a composite score below the threshold at baseline but above the threshold at any time during treatment, and treatment-worsening suicidal ideation (TWOSI), defined as an increase in suicidal-ideation score of at least 0.5 standard deviation above baseline at any time during treatment.
Gender, Drug Affect Risk
Fifty-seven patients (17 percent) met the criterion for TESI at some point during the study, and 197 (42 percent) met the criterion for TWOSI. Among all study participants, neither treatment was significantly associated with the incidence of TESI or TWOSI.
In male patients, however, nortriptyline was associated with significantly higher risk for TESI (OR 9.83, 95 percent confidence interval [CI] 1.72 to 56.27) as well as for TWOSI (OR 2.47, 95 percent CI 1.34 to 4.56) compared with escitalopram.
Other factors, such as greater depression severity, younger onset age, more previous depressive episodes, unemployment, and being married were significant predictors of TESI among patients with no baseline suicidal ideation. Depression severity, history of suicide attempts, and retirement were significant predictors of TWOSI among those with baseline suicidal ideations. The severity of depression during the treatment period was “the strongest predictor of both TESI and TWOSI,” the authors concluded.
Predictive Effect Assessed
The authors also conducted a sensitivity analysis of just the randomized patients and found that the predictive effect of nortriptyline in male patients remained significant for both TESI and TWOSI.
Previous analyses that detected an SSRI-related increase in suicidality were based on placebo-controlled clinical trials conducted to gain new drug approval. The data on suicidal ideation or behaviors had been reported spontaneously, instead of systematically or prospectively. In addition, those clinical trials usually exclude any patient with suicidal ideation or history from enrollment, but they account for a large proportion of patients seen in real clinical settings.
Suicidal Thoughts Decline With Treatment
In the GENDEP trial, more than half (n=473) of the study participants had significant suicidal ideation at baseline, and 102 had a history of suicide attempts. Despite the incidence of TESI and TWOSI, the antidepressant treatment led to continual decline in the mean score of suicidal ideation over the course of the 12-week treatment (see chart). The two treatment groups did not differ significantly after controlling for baseline scores.
In addition, the occurrence of TESI and TWOSI did not skew toward the early days of treatment, but were generally evenly distributed throughout the 12 weeks. “This is of particular concern, as it shows that there is little reason for either intensive monitoring over the first weeks or for complacency later in the course of treatment,” the authors wrote.
Genetic Analyses Point to Neural Pathways
The drug and gender differentiation of TESI and TWOSI incidences were traced to genetic variations among the study patients, according to a separate GENDEP analysis. In a study published in Neuropsychopharmacology (online on July 29 and in the November print issue), the same group of researchers presented results from a genetic predictor analysis of the treatment-related suicidal-ideation data from the same study patients.
Among the 123 polymorphisms in nine candidate genes tested, the three genes BDNF, NTRK2, and ADRA2A were associated with significantly increased suicidal ideation from baseline. The BDNF and NTRK2 genes code for brain-derived neurotrophic factor and its receptor, respectively, suggesting that the BDNF neurochemical pathway is involved in the risk of antidepressant-induced suicidal ideation.
The ADRA2A gene codes for the alpha2A adrenergic receptor, and its polymorphisms are significantly associated with the elevated suicidal-ideation risk in men on nortriptyline, which is consistent with the drug's mode of action in noradrenergic signaling.
According to the GENDEP Web site, researchers hope the insights gleaned from this ambitious project will “lead to the development of a genetic test to assist doctors in choosing the right antidepressants for patients and … advance understanding of the biological mechanisms responsible for an antidepressant being effective.”