Taking placebo for three months in a clinical trial does not cause harm in youth with major depressive disorder, and these young patients respond well to active treatment after the placebo-period ends, according to researchers involved in the Treatment for Adolescents With Depression Study (TADS), a large government-sponsored study.
TADS, funded by the National Institute of Mental Health, began in September 1998 and completed enrollment in March 2003. The active study phase lasted 36 weeks, followed by one year of passive follow-up.
Patients aged 12 to 17 were initially randomized to four treatment groups for 12 weeks: placebo, fluoxetine only, cognitive-behavioral therapy (CBT) only, or the combination of fluoxetine and CBT. The three active-treatment groups continued with treatment for the entire 36 weeks. The analyses of patient outcomes in the placebo group, conducted by Betsy Kennard, Psy.D., of the University of Texas Southwestern Medical Center, and colleagues, were published online in AJP in Advance on January 15.
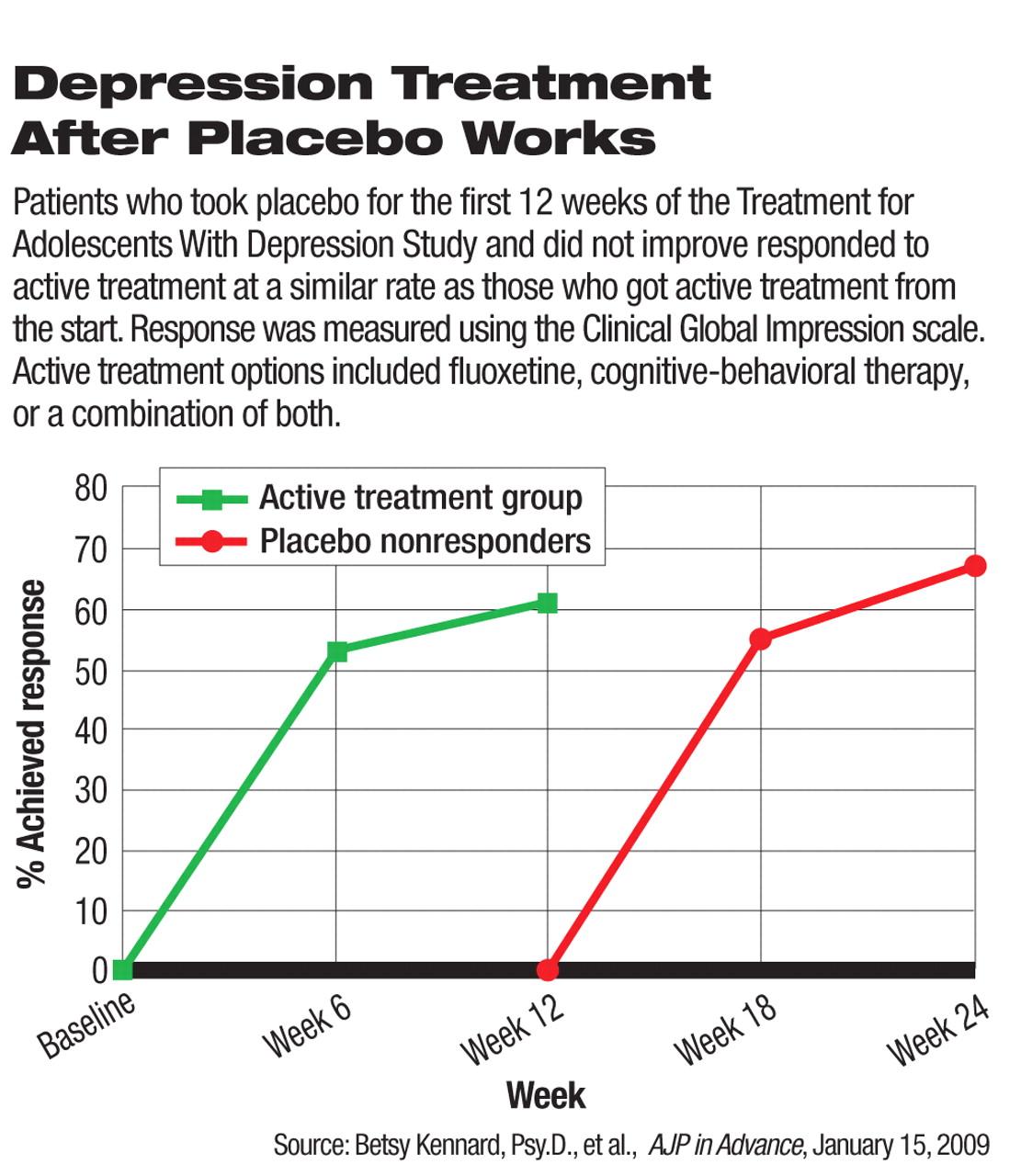
In the placebo group, 112 patients were randomly assigned to placebo treatment in a double-blind manner for the first 12 weeks. At week 12, 65 percent of these patients did not meet the criteria of response and were given the option to receive one of the three active treatments for another 12 weeks. The placebo-responders were given follow-up care and, if they needed treatment, could choose any of the three active treatment options for 12 weeks. In the final 12 weeks, all patients in the placebo group received follow-up and could be actively treated if their depression relapsed.
Response was defined as having a score of 2 or higher on the clinician-rated Clinical Global Impression (CGI) scale.
At week 36, 82 percent of the patients who initially got placebo achieved response as defined by CGI score, which was similar to the 83 percent response rate in the 327 patients who were initially randomized to active treatment for the entire study.
The rate of remission, defined as having a score of 28 or lower on the Children's Depression Rating Scale–Revised, at week 36, was 48 percent in patients who received initial placebo. This was lower than the 59 percent remission rate in the active-treatment patients, but the difference did not reach statistical significance.
Although there had been ethical concerns about undue harm for patients who got placebo for three months without active treatment, patients who responded to placebo during the double-blind period generally stayed well in the follow-up, the authors found, and patients who did not respond to placebo improved with active treatments.
Overall, the patients who were initially put on placebo for 12 weeks“ went on to achieve outcomes similar to those who received active interventions” at week 36, even though the length of active treatment in those who needed it was shorter. The safety risks, such as the rates of worsening depression, suicidal thoughts and action, and side effect–related dropouts, among placebo patients were also not substantially different from those in the other groups.