The hunt for a fast-acting antidepressant moved a step forward with a report of the efficacy of scopolamine, a drug commonly used to treat vertigo, in a clinical trial.
A muscarinic cholinergic receptor antagonist, scopolamine is commonly available in a type of transdermal patch indicated for treating motion sickness. The patch delivers up to one milligram of scopolamine over three days.
To test the drug's effect on depression, Wayne Drevets, M.D., a senior investigator, and Maura Furey, Ph.D., a staff scientist at the Mood and Anxiety Disorders Program of the National Institute of Mental Health, used a dosage of 4 micrograms of scopolamine per kilogram of body weight, given through intravenous infusion.
Twenty-two volunteers with a DSM-IV diagnosis of major depressive disorder were randomized to two regimens: three doses of placebo followed by three doses of scopolamine infusions, or three doses of scopolamine followed by three doses of placebo. Every two infusions were separated by an interval of three to five days.
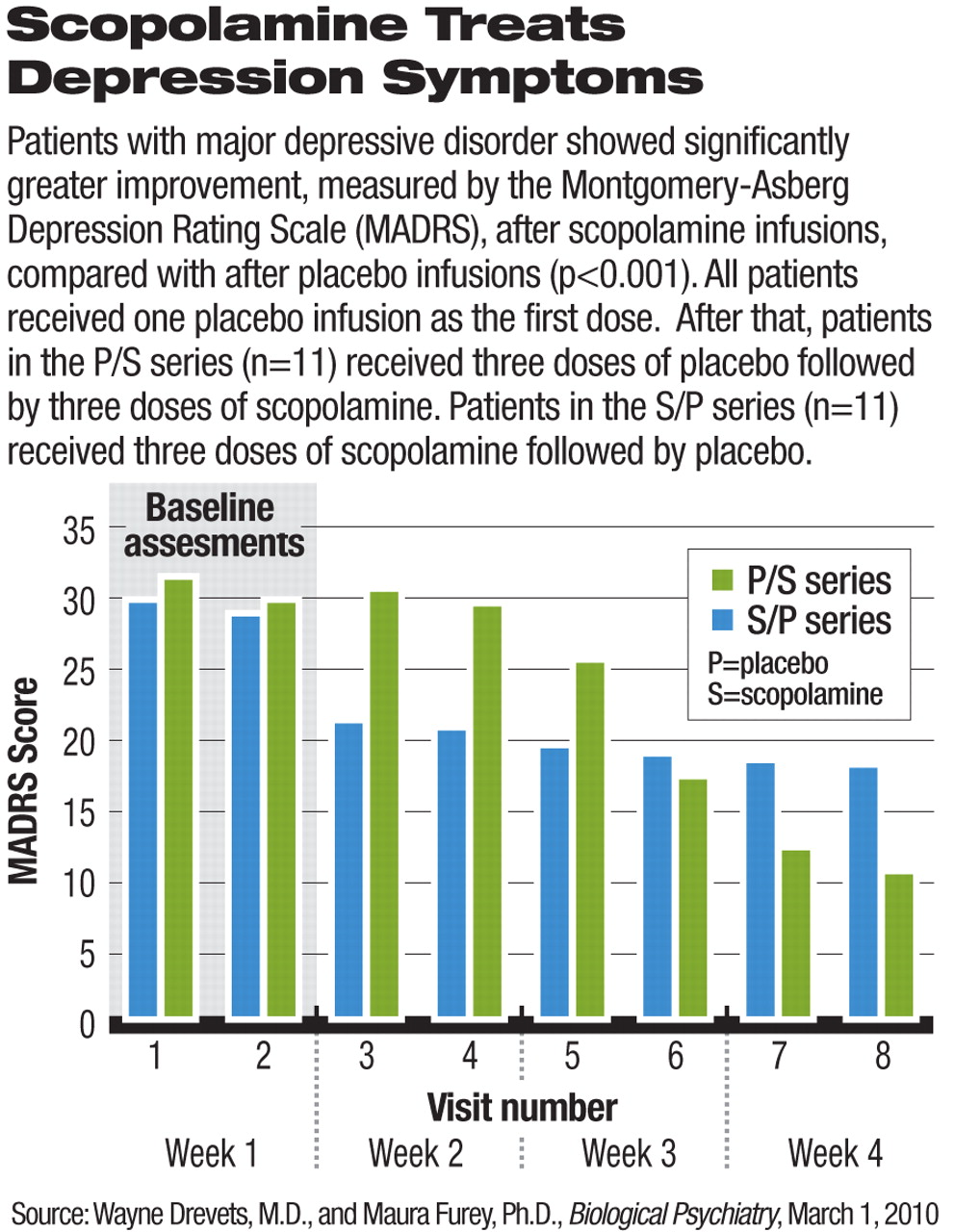
The effect of scopolamine treatment on the patients' depressive symptoms, measured by change in the Montgomery-Asberg Depression Rating Scale (MADRS) score, was significantly larger than the effect of placebo infusion (see chart). Significant reduction in MADRS scores was observed as early as the assessment three to five days after the initial dose of scopolamine.
Among patients who received scopolamine before placebo, the clinical improvement persisted during the placebo phase.
At the end of the study, 14 (64 percent) of the study patients achieved full response, defined as at least 50 percent reduction of MARDS score from baseline. Eleven (50 percent) reached remission, defined as having a MADRS score of 10 or below.
The study participants had moderate to severe depression at baseline, with mean MADRS scores of about 30. Eight participants had comorbid anxiety disorder, and 13 had been in the current depressive episode for more than two years. In addition, six participants had failed to respond to previous treatment.
Furey and Drevets had published a study on the rapid antidepressant effect of scopolamine infusion in the October 2006 Archives of General Psychiatry. In that study, scopolamine significantly reduced depressive symptoms in patients with major depressive disorder and with bipolar disorder. The current study, which replicates previous findings in a group of unipolar depressive patients, was published in the March 1 Biological Psychiatry.
The adverse effects of scopolamine observed in the study were not serious and were expected of anticholinergic drugs. They included drowsiness, blurred vision, dry mouth, light-headedness, and hypotension.
Although the actual mechanism of scopolamine's antidepressant effect is still unknown, the researchers proposed two possible explanations. First, the drug indirectly reduces N-methyl-D-aspartate receptor (NMDAR) in the brain, and NMDAR antagonism is one of the consequences observed with other antidepressant drugs and with electroconvulsive therapy. A second possible explanation is that scopolamine enhances the activity of the parasympathetic nervous system and, in turn, reverses the relatively heightened sympathetic system seen in depression.