Antiepileptic drugs (AEDs) are not associated with an increased risk of suicide attempts in patients with bipolar disorder and may have a protective effect, a large epidemiological study has found.
This study, by Robert Gibbons, Ph.D., and colleagues, runs counter to warnings previously issued by the Food and Drug Administration (FDA) based on the agency's internal analysis, which showed that AEDs double the risk of having suicidal thoughts and behaviors compared with placebo (Psychiatric News, February 6, 2009).
In the current epidemiologic study, after receiving an AED, patients with bipolar disorder had a significant drop in the rate of suicide attempts, from 72 per 1,000 person-years (PY) before the treatment to 13 per 1,000 PY after. The post-AED rate was not significantly different from that of patients with bipolar disorder who received neither an AED nor lithium (also 13 per 1,000 PY). Patients who received lithium monotherapy also had a significant drop in suicide attempt rate from 99 per 1,000 PY before treatment to 18 after treatment.
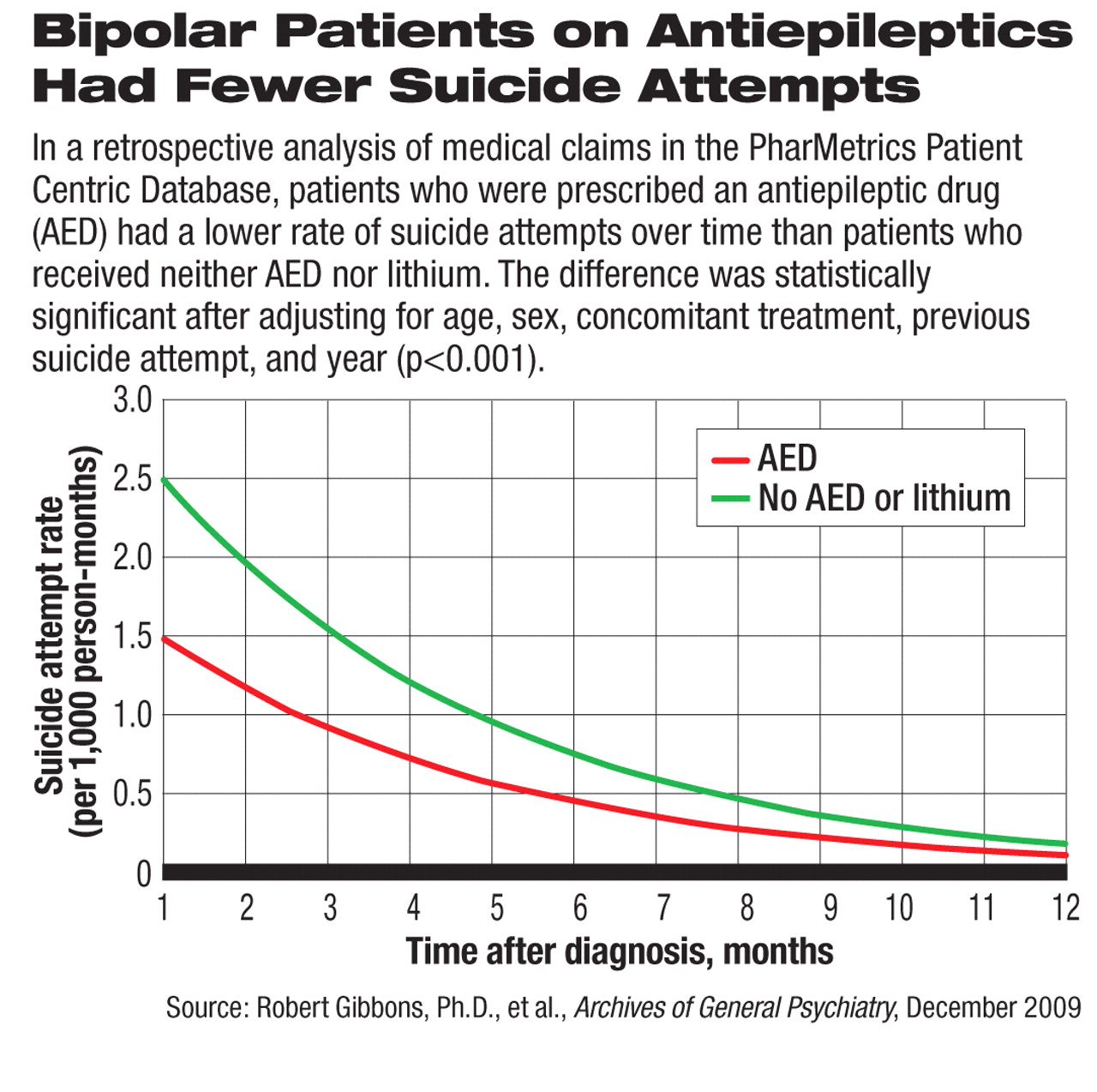
In addition, patients who took only one AED and no other psychoactive drugs had a significantly lower rate of suicide attempts than patients who received no pharmacologic treatment at all (3 versus 15 per 1,000 PY). To account for the natural change in suicide attempt rate over time, the authors analyzed the risk of first suicide attempt after diagnosis as related to AED use on a month-by-month basis (see chart). They found the same significant protective effect of AED treatment compared with patients who did not receive an AED or lithium.
The apparently contradictory conclusions between this study and the FDA's analysis may be related to differences in methodology, the authors acknowledged.
The FDA's analysis was based on pooled data of adverse events reported in randomized, placebo-controlled, double-blind clinical trials involving 11 AEDs, which were not the only AEDs on the market but the only ones with data for comparison with placebo. These drugs have a wide range of mechanisms of action and therapeutic indications, including some with no known efficacy for psychiatric disorders, while others are often used to treat bipolar disorders. Clinical trials for epilepsy, psychiatric disorders, and pain were all combined in the analysis.
The current study, published in the December 2009 Archives of General Psychiatry, was a retrospective analysis of medical claims contained in the PharMetrics Patient Centric Database, a large and nationally representative database that aggregates longitudinal insurance claims from more than 85 managed care plans covering more than 47 million people. The sample for the study consisted of nearly 48,000 patients with a diagnosis of bipolar disorder from 2000 and 2006. The authors chose the same 11 antiepileptics that were included in the FDA analysis.
The study also differed from the FDA analysis in endpoint. The FDA analysis focused on suicidality, which includes suicidal thoughts as well as behaviors. The current study's endpoint was restricted to suicide attempts, which were identified from specific ICD-9 diagnostic codes in the database.
In addition, the FDA analysis compared patients who were randomized to AEDs with those randomized to placebo in drug company–conducted clinical trials, while the epidemiological study compared patients who were prescribed (no randomization) AEDs with those who received either lithium or other drugs such as antipsychotics in “realworld” clinical settings.
The FDA's meta-analysis has been criticized by some researchers, including neurologists, for methodological problems such as combining historical trials with varying drugs, patients, and indications and the lack of prospective, consistent screening method for suicidal thoughts and behaviors.
In addition, “there may be ascertainment bias in placebo-treated patients,” Gibbons told Psychiatric News. For example, if patients in a clinical trial attempted suicide by using the study drug, those who got the active drug would be more likely to end up in the hospital and have a documented suicide attempt than those who got placebo, he explained.
In other words, randomization, blinding, and placebo controls do not entirely eliminate bias in clinical trials.
“Suicide is a very difficult thing to study,” said Gibbons, in part because it is a rare event and thus requires a large base population to conduct meaningful statistical analysis. “It might be the reason for a [psychiatric] diagnosis, a symptom of a disorder, or a side effect of treatment.... It's very difficult to disentangle all these factors,” he noted.
At an advisory meeting in 2008, a panel of outside experts voted in agreement with the agency's conclusion that antiepileptic drugs are associated with increased risk of suicidality, but rejected the agency's recommendation to impose a boxed warning on drug labels (Psychiatric News, August 15, 2008). The agency later mandated a standard, classwide warning about this risk, not boxed, in all AED labels and in a patient medication guide. The mandate was also applied to AED drugs that were not included in their analysis.