Babies born to women with psychotic disorders often have the deck stacked against them. Their mothers tend to receive less prenatal care; have poorer nutrition; use more tobacco, alcohol, and illicit drugs; are unlikely to be married; and often have limited social support. New labeling recently promulgated by the Food and Drug Administration (FDA) warns that these babies may also be susceptible to withdrawal symptoms from their mothers' antipsychotic medications, but some practitioners fear the results of those warnings will be worse than the symptoms of withdrawal.
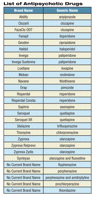
Last month the FDA posted a Safety Alert for Human Medical Products notifying health care professionals that the pregnancy section of drug labels for the entire class of antipsychotic drugs has been updated. The new drug labels contain more and consistent information about the potential risk for extrapyramidal symptoms (EPS) and withdrawal symptoms in newborns whose mothers were treated with antipsychotic medications during the third trimester of pregnancy. Drugs receiving the warning include Haldol, FazaClo, Fanapt, Clozaril, Risperdal, Zyprexa, Seroquel, Abilify, Geodon, Invega, Loxitane, Moban, Navane, Orap, Saphris, Stelazine, Thorazine, and the olanzapine-fluoxetine combination known as Symbyax and are primarily used in the treatment of schizophrenia and bipolar disorder.
The symptoms of EPS and withdrawal in newborns may include agitation, abnormally increased or decreased muscle tone, tremor, sleepiness, severe difficulty breathing, and difficulty in feeding. In some newborns, the symptoms subside within hours or days and do not require specific treatment; other newborns may require longer hospital stays.
According to the FDA, the update was prompted by a search of the FDA's Adverse Event Reporting System database through October 29, 2008, which identified 69 cases of neonatal EPS or withdrawal with all antipsychotic drugs.
"Symptoms reported included agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder," according to the Safety Alert. "Blood levels were not provided, making it not possible to determine whether the events resulted from antipsychotic drug toxicity or withdrawal. Some cases described the time at which the onset of symptoms occurred, and they ranged from birth to one month after birth. The symptoms varied in severity; some neonates recovered within hours or days without specific treatment, while others required intensive care unit support and prolonged hospitalization. Medications used to treat a suspected withdrawal reaction in the neonates included phenobarbital and benzodiazepines."
A majority of the cases were confounded by other factors, including concomitant use of other drugs known to be associated with withdrawal symptoms (antidepressants, benzodiazepines, nonbenzodiazepine hypnotics, alcohol, and opioids), prematurity, congenital malformations, and obstetrical and perinatal complications (placental problems, preeclampsia). However, there were some cases that suggest that neonatal EPS and withdrawal may occur with antipsychotics alone.
But could these new warnings lead medical practitioners to discourage pregnant women from taking antipsychotic medications, and what could be the result of those actions? "The ruling might encourage physicians to refuse these medications to pregnant women for fear of liability and discourage women who do have access from taking them," said former APA President Nada Stotland, M.D., M.P.H.
While the FDA urges practitioners to counsel patients about the benefits and risks of taking antipsychotic drugs during pregnancy and to monitor neonates exhibiting EPS or withdrawal symptoms, Stotland believes too little information has been made available. "There is not nearly enough in the ruling about counseling women to weigh risks versus benefits or about ways to get through the last trimester on no or lower doses of medication. The new labeling does not seem to indicate the level of risk. If it is not high, there is more danger that mothers will see normal newborn behavior as pathological than there is that mothers will recognize EPS in their babies. The whole experience will be colored by guilt over exposing the fetus to the medication."
Previous studies appear to agree. Two Swedish studies by Emma Nilsson, Ph.D., et al., one in 2002 and one in 2008, demonstrated that women with schizophrenia, particularly those women who experience a psychotic episode during pregnancy, are at increased risk for adverse pregnancy outcomes—including stillbirth, fetal death, premature delivery, and neonatal deaths. While these outcomes could be primarily explained by the pregnant women's psychosocial situations, including high rates of smoking during pregnancy and single motherhood, the risk could not be fully explained by other maternal factors.