Vulnerability
Definition of vulnerability. The concept of vulnerability in research is rarely explicitly defined and has been criticized as being too broad to be useful (
26 ). Some have misunderstood it as referring to vulnerability to potential harm through participation in research; however, it typically refers to vulnerability to undue coercion (
2,
27 ). In this respect, individuals with psychiatric disorders have been considered to be potentially vulnerable in two ways: because of their impaired capacity to provide informed consent (
26 ) (capacity-based vulnerability) and because of their susceptibility to coercion as a result of the power differential between the investigator and the potential participant (
1,
28 ) (power-based vulnerability).
Although no formal regulations govern IRBs in the area of vulnerability to coercion in research with persons who have psychiatric disorders, IRBs are strongly urged to pay greater attention to evaluating whether participants have the ability to provide informed consent and to determine what additional safeguards need to be put into place to ensure that participants adequately understand the research procedures, risks, and benefits (
2,
3,
4 ). However, the National Bioethics Advisory Commission (NBAC) report made it clear that although people with psychiatric disorders are considered to be a vulnerable population, not all persons with psychiatric disorders should be considered vulnerable (
2 ).
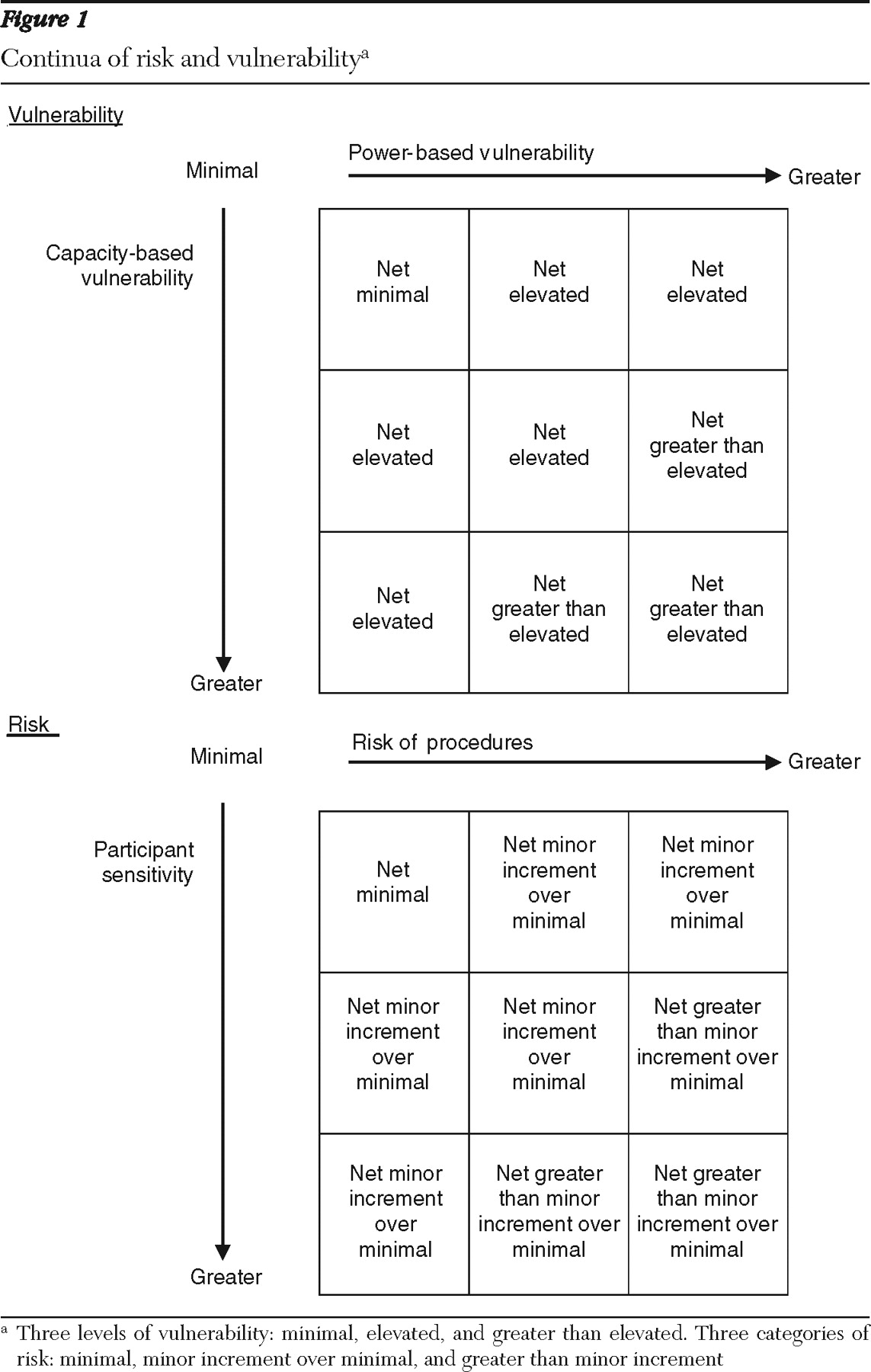
Thus there is believed to be a range of capacity-based vulnerability among persons who have psychiatric disorders. Below we review the evidence for ranges in capacity-based vulnerability in this population and evidence that specific subgroups show a greater degree of impaired capacity than others.
Capacity-based vulnerability. Several studies have documented the degree to which persons with psychiatric disorders (particularly schizophrenia) are able to comprehend informed consent. Dunn's (
24 ) review of research with persons with schizophrenia noted that two major conclusions can be drawn from these studies: that the majority of this population is able to understand the consent process and that on average these individuals show poorer understanding of research than persons without psychiatric disorders. These findings suggest that although diminished capacity is indeed an important consideration in regard to persons who have psychiatric disorders, it nevertheless is not universal or even typical.
We further note that important differences have been observed in the proportion of persons who demonstrate capacity to provide consent, depending on the recruitment source for the study, in particular, whether the sample is inpatient or outpatient. For example, a study with long-term inpatients found that as many as 67% of persons with schizophrenia performed in an inadequate manner on tests of decisional impairment (
22 ); in contrast, two other studies (
20,
21 ) found that only roughly 20%–30% of persons with schizophrenia who were drawn from predominantly outpatient samples showed evidence of decisional impairment.
In addition to the studies conducted with persons with schizophrenia, a small number of studies have been conducted with persons with depressive disorders (
30,
31,
32 ). Appelbaum and colleagues (
30 ) found that more than 90% of participants with major depression demonstrated full comprehension of consent. Similarly, Stiles and colleagues (
31 ) found that participants with depression did not score significantly worse on measures of understanding than participants from the community in a control group but scored better than participants with schizophrenia. Another study of comprehension found that participants with major depression performed somewhat more poorly than community participants but better than participants with schizophrenia and that a majority (over 85%) demonstrated very good comprehension (
32 ). These studies suggest that the capacity of persons with depressive disorders to consent to research is not markedly worse than that of persons in the general population. Although some have suggested that individuals with underlying suicidality may manifest incapacity as a willingness to accept untoward risk (
33 ), no data exist to support this notion.
On the basis of available evidence, we recommend that IRBs recognize that although persons with psychiatric disorders are at risk as a population for vulnerability to coercion, vulnerability is likely to fluctuate with mental state. It is also evident that capacity-based vulnerability to coercion is greater for persons with schizophrenia than for those with major depression (evidence regarding persons with bipolar disorder and other psychotic disorders is lacking). We conclude that capacity-based vulnerability to coercion should be considered a potential state rather than a trait among people with psychiatric disorders and that one way to gauge the likelihood of vulnerability is by determining whether the sample is drawn from persons in the active phase of their disorder, such as when they are in need of hospitalization or crisis services, or in the stable phase, such as when they are in receipt of ongoing services in the community.
Power-based vulnerability. Vulnerability due to a power differential has not been systematically studied among persons with psychiatric disorders. However, a wealth of social psychology literature provides evidence that individuals are generally more susceptible to social influence and to complying with the commands of authority figures when they are placed in institutional settings where their autonomy is restricted (
34 ). In addition to those who are involuntarily hospitalized, the most vulnerable individuals in this respect are those who reside in correctional settings (
35 ). Another consideration with regard to power-based vulnerability is related to cultural characteristics that might lead individuals to be more likely to defer to authority figures. A review of ethical issues related to the involvement of persons from racial-ethnic minority groups in psychiatric research found no studies that directly addressed this issue, but the authors suggested that groups such as unacculturated Asian and Hispanic immigrants might be more vulnerable to "defer" to medical authority when asked to sign informed consent forms (
36 ). It has also been suggested that the need for money to purchase drugs can be a coercive factor in research with individuals who have active substance abuse problems, although research has not explored this issue (
37 ).
We conclude that power-based vulnerability can be assumed to vary and that IRBs can address this issue by considering the setting in which an individual with a psychiatric disorder is approached for research participation, as well as by considering the potential impact of acculturation and the presence of co-occurring active substance abuse.
Risk
Standard definition of risk. Risk is determined by considering both the magnitude of potential harm and the likelihood of potential harm. Although there has been some disagreement over whether research risk encompasses two or three categories, the current predominant opinion is that three risk levels should be considered: minimal risk, minor increment over minimal risk, and greater than minor increment over minimal risk (
6,
38 ). There is a belief that the degree of risk that certain types of studies pose for persons with psychiatric disorders may be different from the degree of risk posed to others (
2 ). Below, we synthesize findings from a variety of studies in order to provide preliminary guidance on the types of studies that can be classified in each of the three major risk categories.
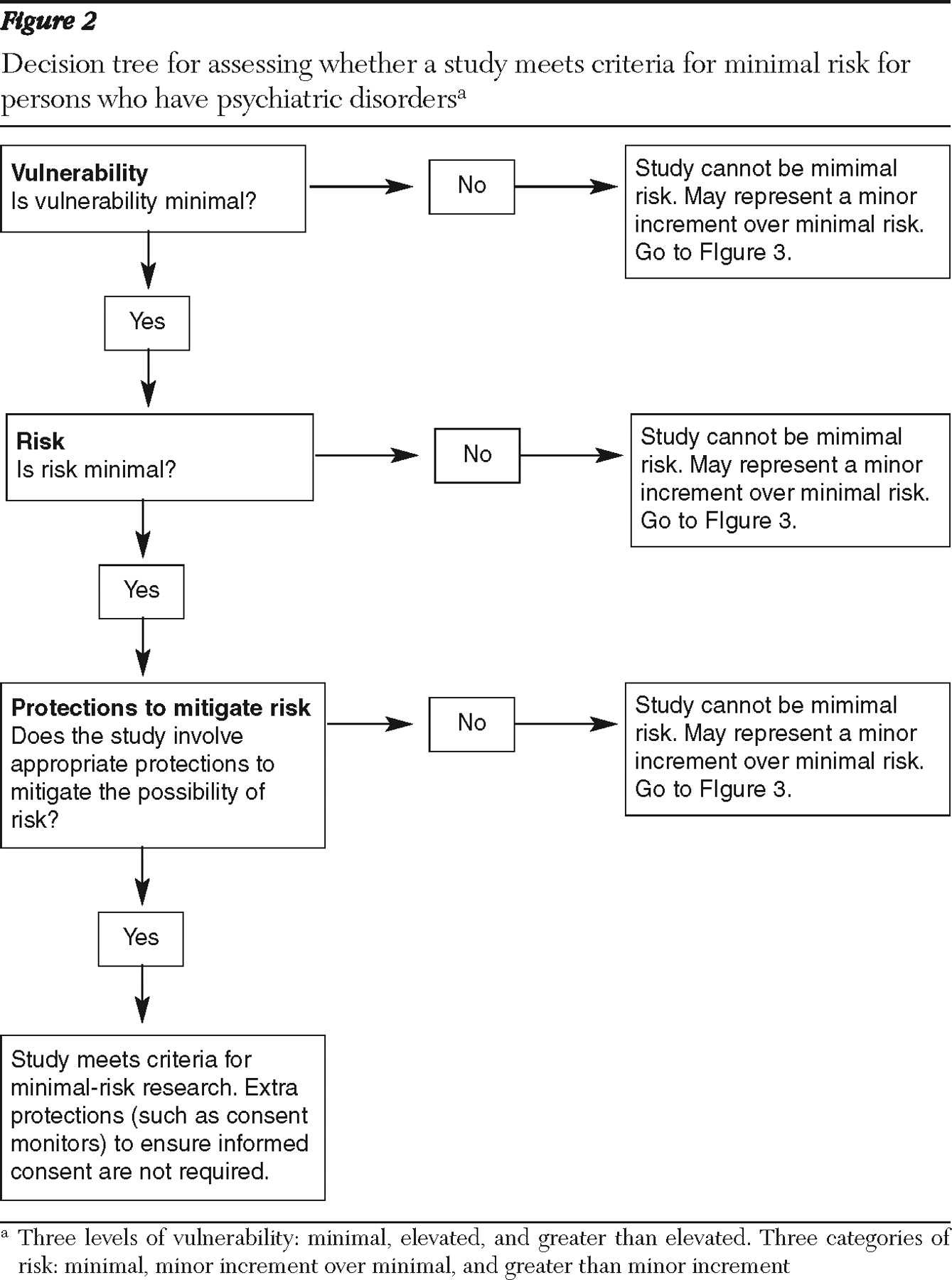
Minimal-risk research. The concept of minimal-risk research was originally drawn from the Common Rule (
4 ) and defined as follows: "the probability and magnitude of harm and discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests." Although there have been disagreements regarding this definition, it has remained the standard by which IRBs evaluate potential risk. NBAC's discussion of the concept of minimal-risk research with persons who have psychiatric disorders implied that some studies might be reasonably judged to fall into this category, but the discussion alerted IRBs to the possibility that there might be "special vulnerability to harm and discomfort" in this population, such that procedures considered to be of minimal risk for persons in the general population might not be considered to be of minimal risk for persons with psychiatric disorders (for example, emotional reactivity might lead persons with psychiatric disorders to become upset by certain questions). There is evidence that IRBs have reacted to this advice by taking a conservative posture, as a matter of course giving studies involving persons with psychiatric disorders a full board review (
39 ). Our review of existing research evidence, however, suggests that this stance may be too restrictive. Below we discuss two common categories of studies that might be considered to be of minimal potential risk for most persons with psychiatric disorders on the basis of available evidence.
Survey- and interview-based studies, which may be considered of minimal risk, are defined here as studies in which persons with psychiatric disorders are asked questions about symptoms, daily life activities, thoughts, feelings, and opinions, either orally or by way of paper-and-pencil questionnaires. Three general categories of studies have examined the risk posed by this type of research: those that retrospectively examined the adverse reactions of persons with psychiatric disorders who participated in such studies (
12,
14 ), those that examined the expected risks of participation as judged by psychiatrists (
15,
16,
17 ), and those that examined the expected risks as judged by persons with psychiatric disorders (
15,
16,
17,
18 ).
Findings from the retrospective studies indicated that less than 10% of persons with psychiatric disorders who participated in interview-based research experienced more than minimal anxiety (
12,
14 ) and that even fewer reported that this anxiety reached "severe" levels. Comparable rates of reports by participants of greater than minimal anxiety have been found in behavioral studies of psychiatric symptoms in populations without psychiatric disorders (
40,
42 ). These findings suggest that adverse reactions are unlikely, that they occur among people with psychiatric disorders only slightly more often than in the general population, and that they reach severe levels very rarely (if ever). Studies of psychiatrists have found that they regard questionnaire-based studies as the least risky category of research for people with psychiatric disorders and that they consider these studies to involve less risk than persons in this population would encounter in daily life (
16,
17 ). Findings from the third category of studies have found similar evidence. Persons with schizophrenia viewed questionnaire-based research as less risky than daily life and as the least risky type of research overall; a low rating of risk was related to their potential willingness to participate in such studies (
19,
41 ).
Routine physical examinations may also be considered to be of minimal risk. Two categories of studies have examined the risk posed by this type of research: those that examined the expected risks of participation as judged by psychiatrists (
15,
16 ) and those that examined the expected risks as judged by persons with psychiatric disorders (
41 ). The first category of studies found that psychiatrists consistently rated research involving routine physical examination procedures as among the least risky categories of research for people with psychiatric disorders and that they considered that the risk involved is less than the risk that persons in this population would encounter in daily life (
15,
16 ). Similarly, the second category of studies found that persons with schizophrenia viewed research involving physical examination procedures as less risky than daily life and among the least risky types of research (
17 ).
On the basis of the existing evidence, we conclude that both the probability and the magnitude of risk posed by survey- and interview-based studies and by studies that involve routine physical examinations and blood draws are small when conducted with persons who have psychiatric disorders. This conclusion is consistent with the general consensus that such research poses minimal risk when conducted with persons in the general population.
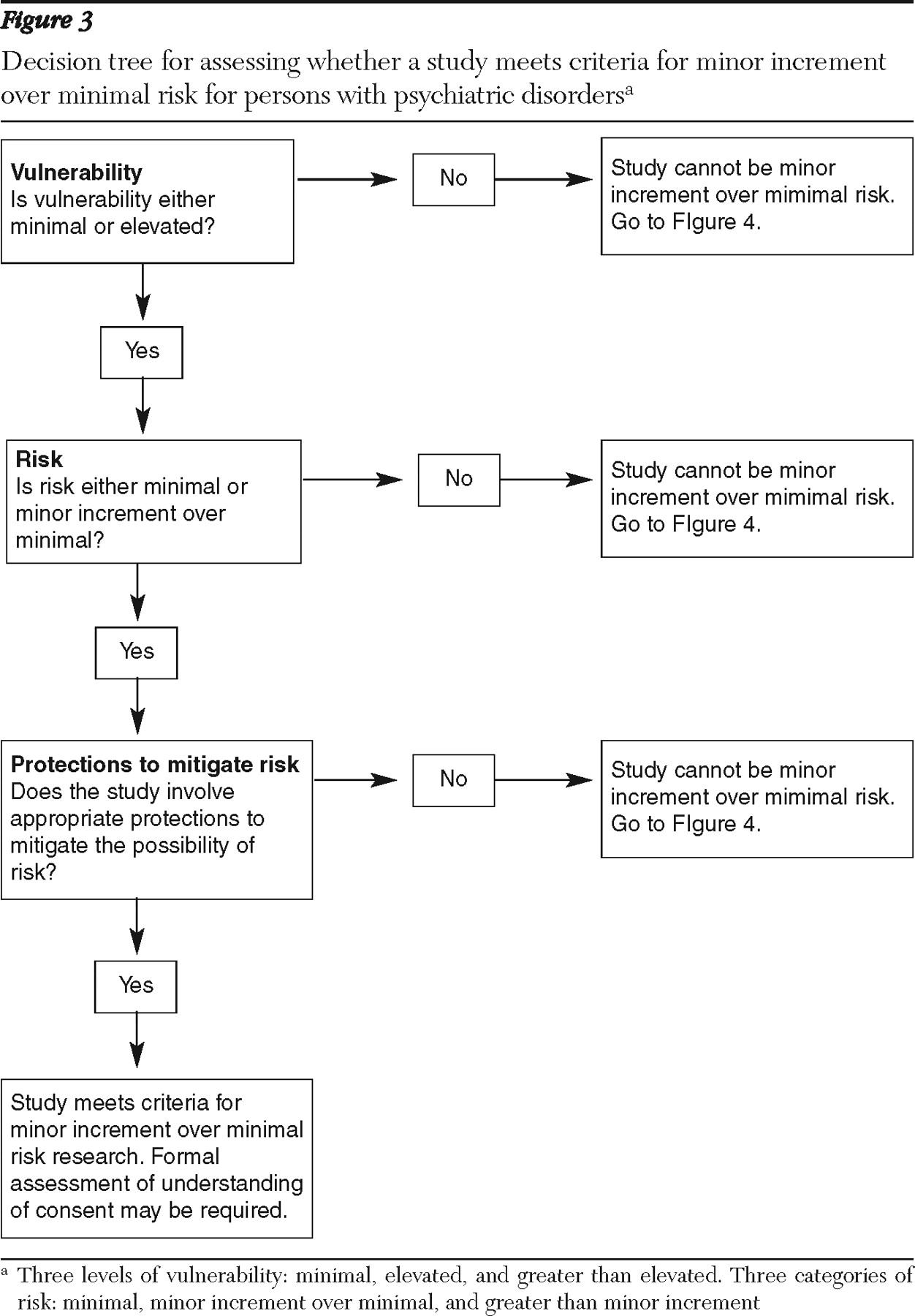
Minor increment over minimal risk. Minor increment over minimal risk is a category that exists in the federal regulations for research with children and has not been officially adopted in any regulations pertaining to adults. However, the category is commonly applied to research with vulnerable adults. A work group commissioned by the New York State Department of Health defined this category as consisting of studies in which "the probability and magnitude of harm … are only slightly greater … than those ordinarily encountered during the performance of routine physical or psychological examinations or tests" (
43 ). Thus this category may include types of research in which the risks of harm occur slightly more frequently or are slightly more serious than those in the studies described above as being of minimal risk. Below we discuss two common categories of studies that might be considered to involve minor increment over minimal risk for most persons with psychiatric disorders on the basis of available evidence.
Some procedures involving physical examinations or medication administration may involve a minor increment over minimal risk. Two categories of studies have examined the risk posed by this type of research: those that examined the expected risks of participation as judged by psychiatrists (
15,
16 ) and those that examined the expected risks as judged by persons with psychiatric disorders (
17,
41 ). The first category of studies found that psychiatrists consistently rated studies involving more invasive physical procedures, such as undergoing an MRI with sedation or taking a new experimental medication, as a moderately risky category of research for people with psychiatric disorders and that they considered that the risk involved is less than or equal to the daily risks encountered by persons in this population (
15,
16 ). The second category of studies found that persons with schizophrenia viewed research involving these physical procedures to be about as risky as daily life and moderately more risky than the procedures that we have characterized as minimal-risk research (
17,
41 ).
Behavioral research on provocative topics may also involve a minor increment over minimal risk. Despite the evidence previously presented regarding the minimal risk of survey- and interview-based research, there is evidence that studies of provocative topics may present a greater likelihood and a greater magnitude of adverse reaction (
13 ). Studies that specifically focus on traumatic stress, such as childhood sexual abuse and adult trauma and victimization, may be of special concern, especially when conducted with individuals who have experienced trauma. Our review found a greater likelihood of intense distress in such studies; in particular, a study conducted with psychiatric inpatients found that 24% became very upset as a result of participation (
13 ). Similarly, research has found that persons meeting criteria for psychiatric disorders such as posttraumatic stress disorder (PTSD) and major depressive disorder were more likely to report experiencing emotional reactions in response to a "lengthy and intrusive" interview that included questions about trauma and victimization (
19 ). However, the participants also reported that they did not regret participating. The authors of a recent review similarly concurred that there is greater evidence for distress in trauma-focused studies than in other behavioral research with persons who have psychiatric disorders (
14 ).
Concerns have also been raised about research on other types of "provocative topics," such as studies involving violent or sexually charged imagery or descriptions, which may pose greater emotional risks (
44 ). Although no research has examined reactions to such studies among persons with psychiatric disorders, persons with schizophrenia rated the expected risk of research that involved viewing an "upsetting image" as riskier that other types of survey research (
41 ).
On the basis of the existing evidence, we conclude that more invasive physical procedures, such as an MRI with sedation and a trial of a new medication, as well as interviews about sensitive topics, can be reasonably expected to pose a minor increment over minimal risk among persons with psychiatric disorders.
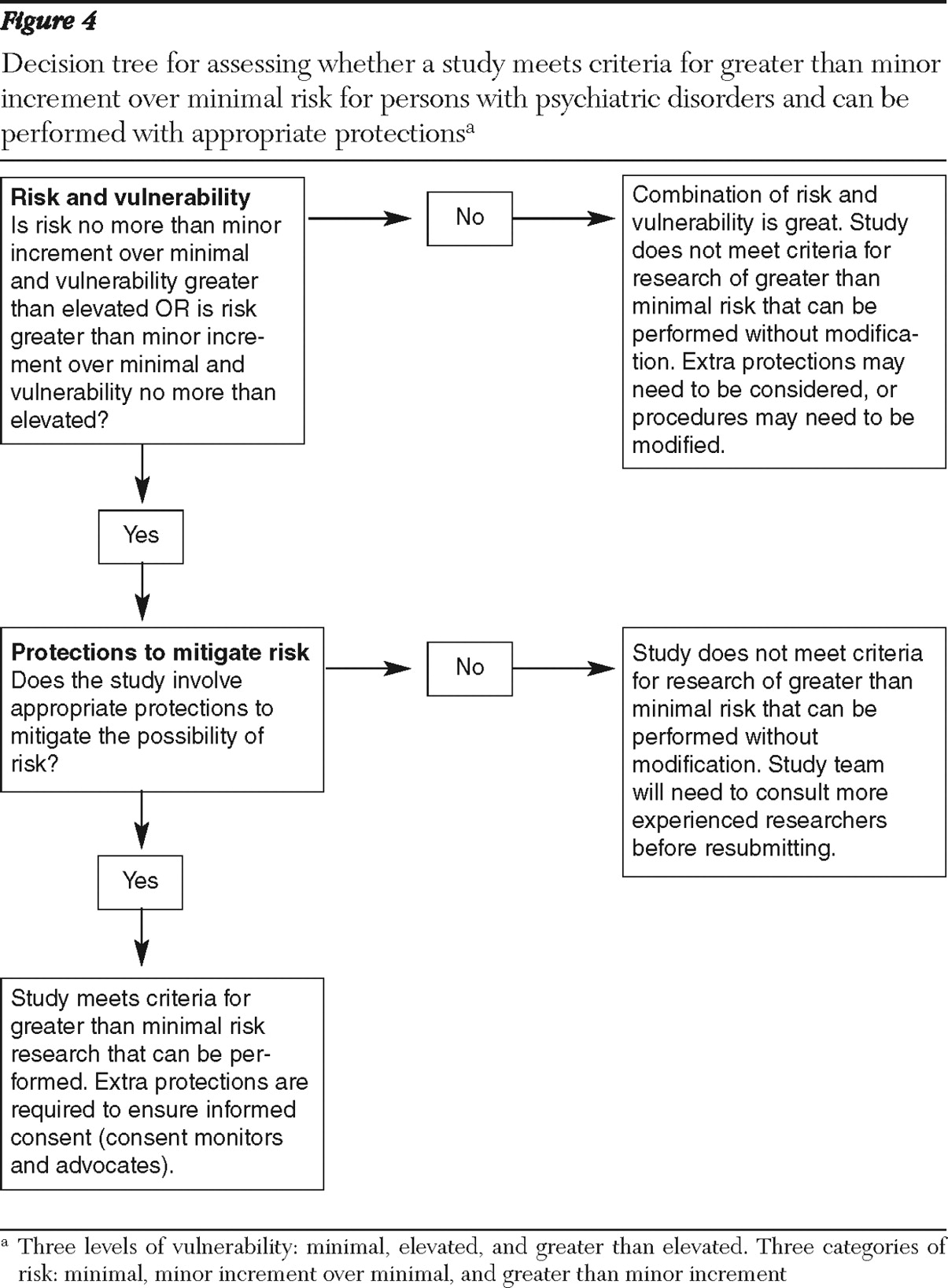
Greater than minor increment over minimal risk. No clear definition exists for evaluating greater than minor increment over minimal risk in federal regulations. However, one IRB offered a useful definition, characterizing such studies as those in which either the probability of risk is likely (harm occurs for greater than 30% of participants) or the magnitude of risk is severe, even given a low frequency of occurrence (
45 ).
Examples of study types that may fall into this risk category are medication washout studies, placebo-controlled medication studies, and symptom challenge studies. Shamoo (
46 ) has expressed strong concerns for the appropriateness of these procedures. Some mental health consumers have also expressed their hesitancy to participate in these types of studies. Consumers who responded to a survey about their reasons for not participating in research gave the following reasons: the obligation to switch medications, the length of time required for stabilization, and lack of knowledge about whether the provided drug was a placebo (
47 ). In previous studies psychiatrists have consistently rated three types of studies as riskier than the daily risks encountered by persons with psychiatric disorders: those that involve more than a two-day medication washout, those in which patients receive a medication that causes symptoms, and those in which patients receive a placebo instead of a medication (
15,
16 ). Studies that included persons with schizophrenia indicated that this population has similar views of research involving these physical procedures, rating them as riskier than daily life and significantly riskier than the studies characterized above as involving minor increment over minimal risk (
17,
41 ).
Some data are available on the actual risks of these types of studies. Wyatt and colleagues (
48 ) examined the long-term effects of participating in conditions that involved placebo among 127 individuals with schizophrenia who were involved in medication trials conducted by the National Institute of Mental Health. All individuals who had been placed on placebo were eventually treated with antipsychotic medication. This research indicated that although all individuals who had received the placebo eventually returned to a baseline level of symptoms, the duration of their recovery period was typically six weeks after the restoration of medication. Although the eventual stability of the participants suggests that placebo-controlled studies do not cause irreparable harm, the length of time required to return to baseline suggests that they experienced a significant loss of functionality for a substantial period. Thus placebo-controlled studies may cause a great delay in the return to stability among persons with schizophrenia and the delay should be considered a risk of such research.
With regard to washout studies, Jeste and colleagues (
49 ) reviewed relapse rates of more than 3,000 persons with schizophrenia who were "withdrawn" from antipsychotic medication in research trials. By 24 months, the relapse rate for these individuals was more than twice the rate for persons "maintained" on antipsychotic medications (59% compared with less than 27%). On the basis of these findings, it was recommended that, whenever possible, medication studies should not use lengthy washout periods but instead taper individuals off of previous medications in order to transition to new medications and, whenever possible, conduct studies in an inpatient environment to protect against harm associated with relapse. Grunebaum and colleagues (
50 ) found that short-term medication washout from antidepressants and mood stabilizers (roughly two weeks) posed few risks for persons with major depressive disorder while they were hospitalized. Risks of relapse may be less of a concern for short-term washout from antidepressants.
As previously stated, symptom challenge studies have been heavily criticized by Shamoo (
46 ), who reviewed studies involving the use of amphetamines and other chemicals to trigger symptoms among persons with PTSD and schizophrenia, stating that such studies compromise the psychiatric stability of those participating in them. However, he provided little empirical data in his discussion of the effects of these studies. Lahti and colleagues (
51 ), however, reviewed the long-term outcome of 25 persons with schizophrenia who participated in ketamine challenge studies and found no support for serious adverse effects over the course of eight months. Carpenter (
52 ) also reviewed several ketamine challenge studies and found that only three patients terminated participation in the research because of discomfort (two from the study group and one from the placebo group). He concluded that there was no evidence that ketamine induction led to a relapse. Data from studies of patients with disorders other than schizophrenia are not available. Furthermore, few data are available on the extent to which other challenge studies might create risk of relapse.
On the basis of the available data, we conclude that studies that involve placebo control, lengthy medication withdrawal, and symptom challenge present risks that are potentially of greater frequency or severity than the studies described above as involving minor increment over minimal risk. Specifically, although the effects of these procedures may be mitigated in the long term, participants with some psychiatric disorders who participate in placebo studies and withdrawal studies may be likely to experience more severe symptoms for a longer period, which may have an impact on their functioning. Although challenge studies are controversial, no evidence of long-term effects has been found for the majority of participants, but some studies have suggested an increased risk in rare cases, which may constitute a greater than minor increment over minimal risk.