Because of recent rapid inflation in expenditures for prescription drugs, especially psychoactive medications, state Medicaid programs have increasingly relied on prior authorization to control Medicaid drug spending. A 2005 survey of 36 states and the District of Columbia found that all had attempted to control Medicaid drug costs by requiring prior authorization for some medications and that more than one-third of Medicaid programs and Medicare Part D plans required prior authorization for one or more second-generation antipsychotic medications (
1,
2,
3,
4 ). A prior-authorization program requires physicians to obtain special approval before they can prescribe restricted (nonpreferred) medications. Prior-authorization policies have been shown to be very effective at reducing pharmacy expenditures while not increasing adverse outcomes when applied to drug classes in which drugs are highly substitutable and more expensive drugs are not necessarily more effective (
5,
6,
7 ). However, the economic and clinical effects of prior-authorization policies for essential psychiatric medications are poorly understood, especially for vulnerable, low-income beneficiaries with bipolar disorder.
Bipolar disorder affects 2.6% of the American general population age 18 and older in any given year and costs $45 billion per year (including direct medical costs and indirect economic costs) in the United States (
8,
9 ). Primary treatment for bipolar disorder includes traditional mood stabilizers, second-generation antipsychotics, and anticonvulsant agents. Drug therapy for bipolar disorder is effective in controlling both manic and depressive episodes and in preventing relapses, hospital admissions, and institutionalization (
10,
11 ). Patients who prematurely discontinue treatment have higher costs because of increased rates of functional impairment, rehospitalization, full-episode recurrences, and suicide (
12 ). Response to drug therapy may differ, depending on the severity of an episode; presence of comorbid psychiatric disorders, such as substance abuse, anxiety disorders, or attention-deficit hyperactivity disorder; presence of general medical conditions, such as kidney insufficiency; demographic factors, including family supports; and personal history of response to a particular agent (
13 ).
In July 2003 the Maine Medicaid program instituted a prior-authorization policy affecting new prescriptions for nonpreferred agents used to treat bipolar disorder. This policy affected only initial prescriptions and not refills. Two second-generation antipsychotics, olanzapine and aripiprazole, were designated as nonpreferred drugs that required prior authorization before dispensing. In addition, for this drug class only, physicians could prescribe subsequent preferred agents—ziprasidone and quetiapine—after failure of an initial preferred agent (risperidone). Among anticonvulsants, lamotrigine, topiramate, gabapentin, brand-name carbamazepine, brand-name valproic acid, oxcarbazepine, and levetiracetam were listed as nonpreferred. The prior-authorization requirement for second-generation antipsychotics was discontinued in March 2004, eight months after policy implementation, although the requirement remains in effect for newer anticonvulsants. The Maine prior-authorization policy permitted physicians to prescribe nonpreferred medications only after failure of a preferred agent used at full therapeutic dosages for at least two weeks or after submitting a form requesting prior authorization by documenting (with supporting office notes) medical necessity for the nonpreferred medication.
The prior-authorization policy in Maine for new antipsychotic and anticonvulsant medications may have changed treatment patterns among patients newly treated for bipolar disorder. First, to avoid the hassle of the prior-authorization process, physicians may have been more likely to prescribe the preferred agents, resulting in a population-level shift in treatment toward preferred agents. However, in a previous study of patients with schizophrenia, we found that the Maine policy was also associated with higher risk of therapy discontinuity (specifically, gaps in use and switching medications) (
14 ). We hypothesized that the policy may have led to higher rates of treatment discontinuity if administrative problems created unintended barriers to refilling medications or if patients taking preferred medications experienced side effects or other undesirable outcomes.
In this study we conducted an evaluation with a strong quasi-experimental design of the real-world effects of prior authorization on market share, treatment discontinuation, and spending for second-generation antipsychotic and anticonvulsant agents for patients with bipolar illness. On the basis of the results of our previous study, we hypothesized that the policy would result in a shift toward prescribing newer agents and increased rates of disruptions in therapy among patients newly treated during the policy period.
Methods
Medicaid and Medicare data
Using data from a previous study of schizophrenia, we analyzed complete Medicaid claims extracted from the Medicaid Statistical Information System for 2001–2004 for Maine (the study state) and New Hampshire (comparison state) (
14 ). If patients were concurrently enrolled in Medicare, we obtained their Medicare claims and enrollment data and linked them to Medicaid claims via a unique patient identifier used in both systems (
14 ). For all patients, Medicaid was the primary source of pharmacy claims data. Pharmacy claims contained patient-identifying information, the National Drug Code, the prescription-filled date, the number of units provided (number of tablets, for example), days' supply, and amount reimbursed.
The Harvard Pilgrim Health Care Institutional Review Board approved the study, waiving consent because our study was conducted with deidentified patient data from a large administrative claims data set.
Study cohorts
Continuously enrolled cohorts. We identified patients who were continuously enrolled in Maine or New Hampshire Medicaid during the study period (January 2001 to December 2004), who were age 18 or older in 2001, and who had at least one inpatient or two outpatient diagnoses of bipolar disorder (
ICD-9-CM codes 296.0, 296.1, 296.4–296.7, 296.89, and 301.11) during the study period (
14 ). There were 6,712 patients (5,336 in Maine and 1,376 in New Hampshire) in the continuously enrolled cohorts.
Newly treated cohorts. The prior-authorization policy implemented in Maine Medicaid on July 1, 2003, was intended to affect new prescriptions of nonpreferred drugs. We therefore identified all patients who were newly treated with any bipolar medications. As in our previous study, we required newly treated patients to have had no use of bipolar medications in the 90 days before the initial prescription was filled (
14 ). To examine the policy impact, we identified two cohorts of newly treated patients in each state. We defined the "policy cohort" as patients who initiated any bipolar medication between July 1, 2003, and February 29, 2004 (Maine, 946 patients; New Hampshire, 133 patients). We defined the "prepolicy cohort" as those who initiated bipolar medication between July 1, 2002, and February 28, 2003, the same calendar period one year before implementation of the policy (Maine, 1,014 patients; New Hampshire, 133 patients). A small proportion of beneficiaries were in both the prepolicy and policy cohorts (60, or 6% in Maine and seven, or 5% in New Hampshire).
We defined the index date as the date of the first prescription filled. We required newly treated patients to be continuously enrolled for eight months before and eight months after the index date. They also were required to meet the same age and diagnostic criteria as the continuously enrolled cohort.
Outcome measures
Bipolar drug utilization and expenditures. For the continuously enrolled cohort, we created measures of prevalence of medication use and cost. Prevalence of use of nonpreferred and preferred medications per month was defined by distributing filled prescriptions over time according to their days' supply and then calculating the proportion of patients who had any bipolar drug use in each month.
To determine changes in Medicaid pharmacy spending for bipolar treatments, we used previously validated methods to calculate the average pharmacy reimbursements per person per month (
14 ).
Prior authorization and discontinuation of bipolar medication. We defined discontinuation as having a gap in use of any bipolar medication of at least 30 days, measured from the time the days' supply from all prior prescriptions filled had been allocated until the time of the subsequent prescription (
14 ). The day of discontinuation was defined as the day after the last day of treatment availability (preceding the gap of 30 or more days). Shorter times until discontinuation would suggest an increased rate of disruption in treatment.
Switching and augmentation of the initial drug regimen. We identified patients who initiated only one drug (rather than multiple drugs) in a specific therapeutic category affected by prior authorization. Switching was defined as a change from the initial drug regimen. Augmentation was defined as filling prescriptions for additional medications used for bipolar illness (lithium and second-generation antipsychotic and anticonvulsant agents) after the initiation of bipolar drug therapy.
Statistical analysis
We used segmented time-series regression models to examine the impact of the prior-authorization policy on the prevalence of use of preferred versus nonpreferred bipolar medications and their associated costs in the continuously enrolled cohort. The prior-authorization policy for second-generation antipsychotic agents was implemented for only eight months. To demonstrate the policy impact, we created three eight-month segments: the prepolicy period (November 1, 2002, through June 30, 2003), the policy period (July 1, 2003, through February 29, 2004), and the postpolicy period (March 1, 2004, through October 31, 2004). We used the same three segments to estimate the impact of prior authorization on the use of anticonvulsants as well, but during the last period (March 1, 2004, through October 31, 2004) a prior-authorization policy was still in effect for anticonvulsants. This difference allowed us to compare the effects of prior authorization for second-generation antipsychotics and anticonvulsants. The time-series models estimated changes in levels and monthly trends of medication use and expenditures for each segment and for both study and comparison groups. In the time-series regression, we controlled for all significant autocorrelation terms and excluded nonsignificant (p≥.10) time-series terms step by step (
15 ).
We used extended Cox regression models to examine the impact of the prior-authorization policy on hazard rates of treatment discontinuation and medication switching or augmentation in the newly treated prepolicy and policy cohorts. We calculated the relative ratios of hazard rates between the policy and prepolicy cohorts of newly treated patients in Maine and compared them with the relative ratios in New Hampshire. In the extended Cox hazards model, we included indicators for policy, state, and the policy-state interaction term (a "difference-in-difference" estimate). We controlled for age, gender, and dual enrollment in Medicaid and Medicare. We also controlled for two comorbidity measures: the number of nonbipolar medications dispensed and the number of inpatient admissions (
16 ).
In the outpatient setting, medications are often prescribed on a monthly basis. We used days' supply indicated on the claims as a proxy for actual drug utilization. Because patients typically receive a 30-day supply of medications, we could not identify the exact date of discontinuation within a 30-day period. For example, even if a patient stopped taking medicines one week after the initiation of therapy, she or he would be categorized as having discontinued on day 31 after initiation of therapy, rather than on day 8. As a result, there was a cluster of discontinuations at 30 days' postinitiation of therapy. To address this limitation, we allowed the baseline hazard rates of discontinuation for those who discontinued drug therapy more than 30 days after initiation of therapy to differ from the baseline hazard rates of those who discontinued at 30 or fewer days. This adjustment was made by using an extended Cox hazard model, in which we stratified patients according to the timing of discontinuation (≤30 days versus >30 days).
Results
Background characteristics of study cohorts
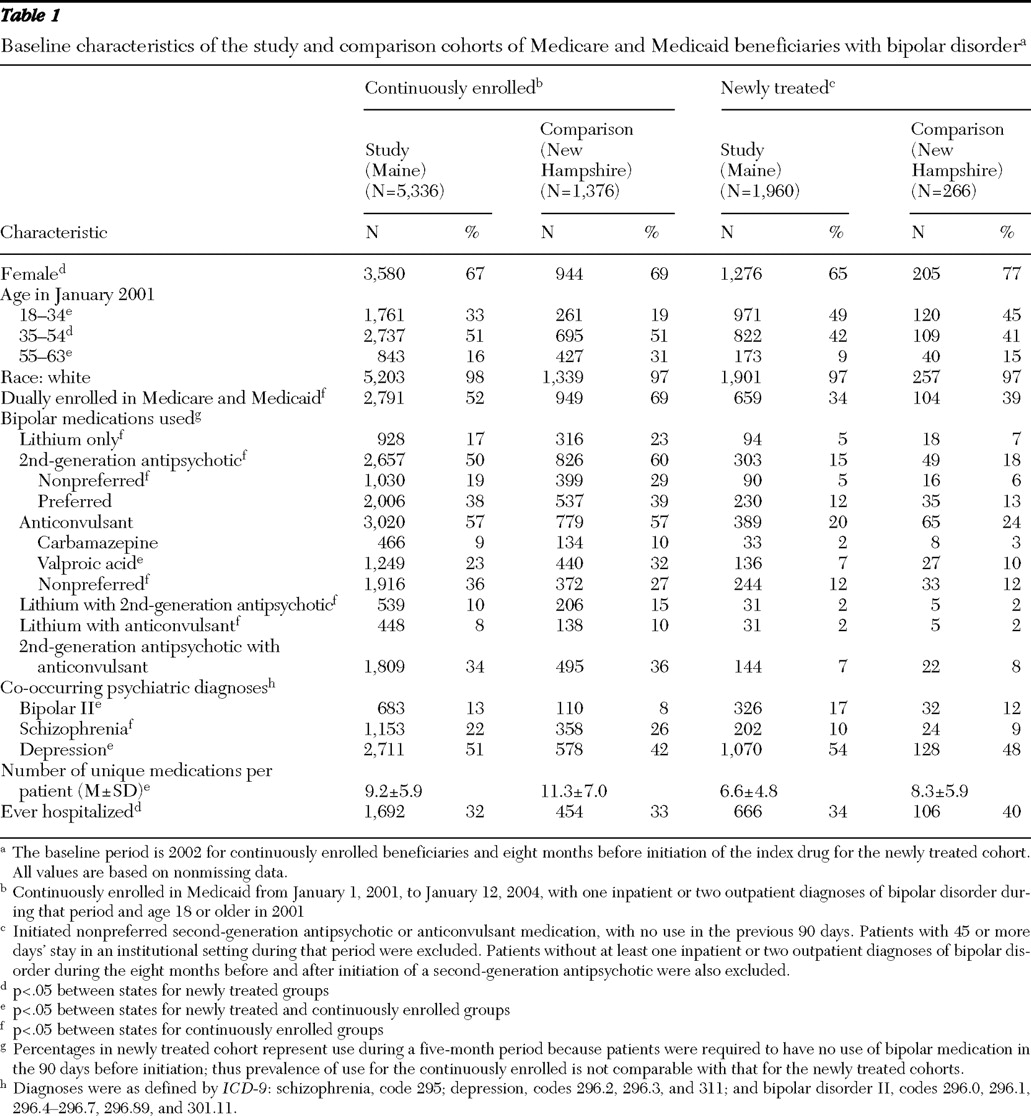
Most demographic and utilization characteristics of enrollees were comparable during the baseline period between Maine and New Hampshire (
Table 1 ). In both states, continuously enrolled patients were more likely to be female than male (67% in Maine and 69% in New Hampshire), 51% were between the ages of 35 and 54, about 38% had used a preferred second-generation antipsychotic, 57% used an anticonvulsant agent, and the lifetime rate of hospitalization was about 32%. However, Maine patients were younger than New Hampshire patients (33% aged 18–34 in Maine, compared with 19% in New Hampshire), and a greater proportion of patients in New Hampshire were dually enrolled in Medicaid and Medicare. Half of the Maine patients used a second-generation antipsychotic agent, compared with 60% in New Hampshire. Seventeen percent of Maine patients used solely lithium at baseline, compared with 23% of New Hampshire patients. Combinations of drug treatment were common (
Table 1 ).
For newly treated patients, baseline drug utilization patterns were similar between the study and comparison groups. Sixty-five percent of Maine patients were female, compared with 77% of New Hampshire patients. Slightly more than 40% of patients in both states were aged 35 to 54. At baseline, 34% of Maine patients had ever been hospitalized, compared with 40% of New Hampshire patients.
Effects of prior authorization on use of medications
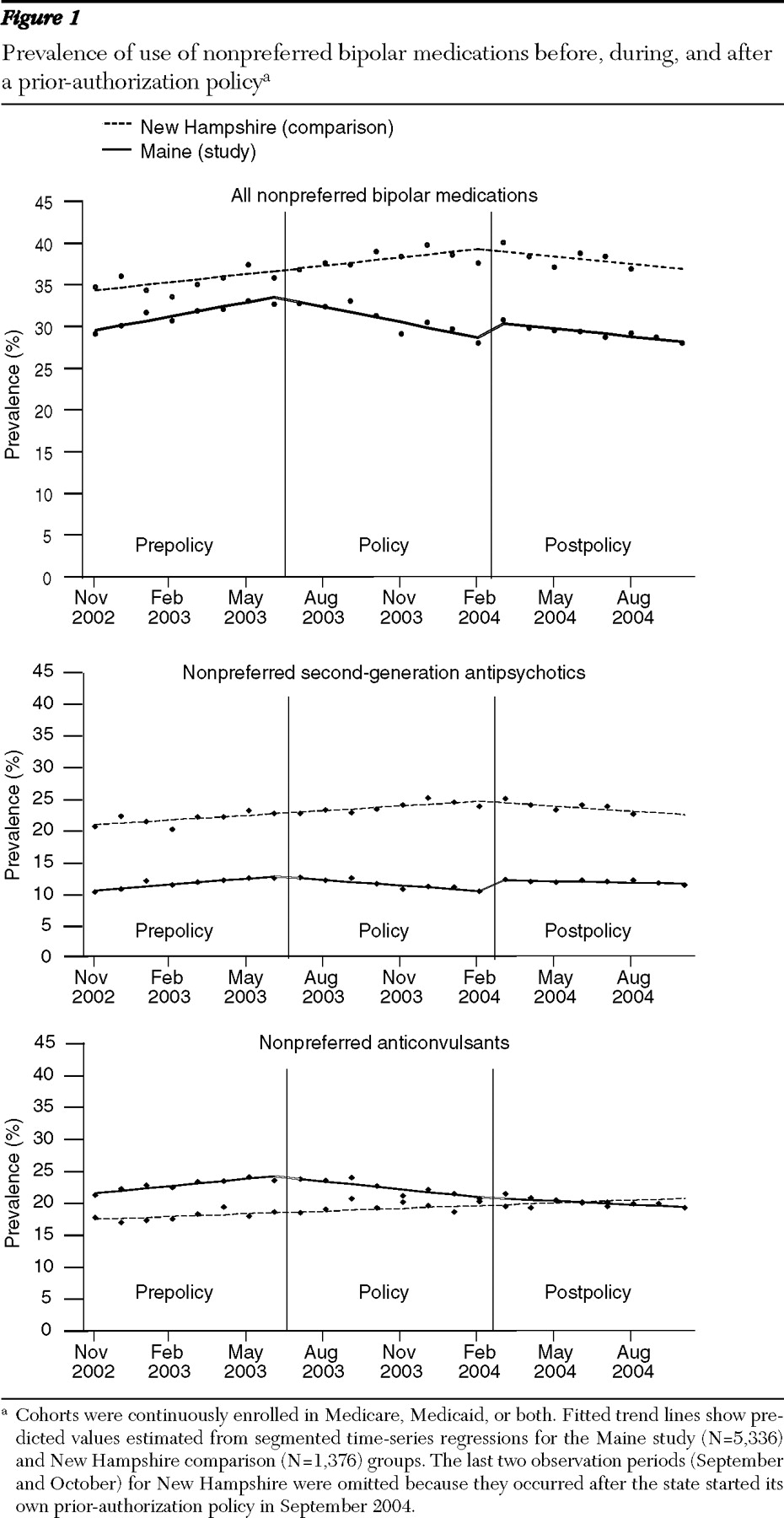
Before the implementation of the prior-authorization policy, the prevalence of use of all nonpreferred medications (specifically, second-generation antipsychotics and anticonvulsants that later required prior authorization) among four-year continuous enrollees was 30% in Maine, with a small upward trend of .05% per month, and 34% in New Hampshire with a similar upward trend (
Figure 1, top). During the eight-month policy period (July 2003–February 2004), the upward trend in the use of nonpreferred medications reversed in Maine, yet the upward trend remained unchanged in New Hampshire. When we used the trend before the prior-authorization period as a counterfactual and controlled for secular trends suggested by the comparison state, the prior-authorization policy was associated with an 8-point decrease in the prevalence of use of nonpreferred bipolar drugs over the eight-month policy period. After the prior-authorization policy ended for second-generation antipsychotic agents, the downward trend in the prevalence of use of nonpreferred second-generation antipsychotics stopped, and the prevalence of use remained stable during the postpolicy period (March 2004–October 2004) (
Figure 1, middle). However, the downward trend in use of nonpreferred anticonvulsants persisted (
Figure 1, bottom).
The prevalence of use of preferred medications was stable at 41% in Maine and 55% in New Hampshire before the policy intervention. During the eight-month policy period, the prevalence of use of preferred drugs increased by 1% in both states. After the policy was repealed, we observed a slight decrease in use of preferred drugs in Maine of 1% during eight months, whereas use of preferred drugs in New Hampshire remained stable.
Pharmacy spending by Medicaid for bipolar medications
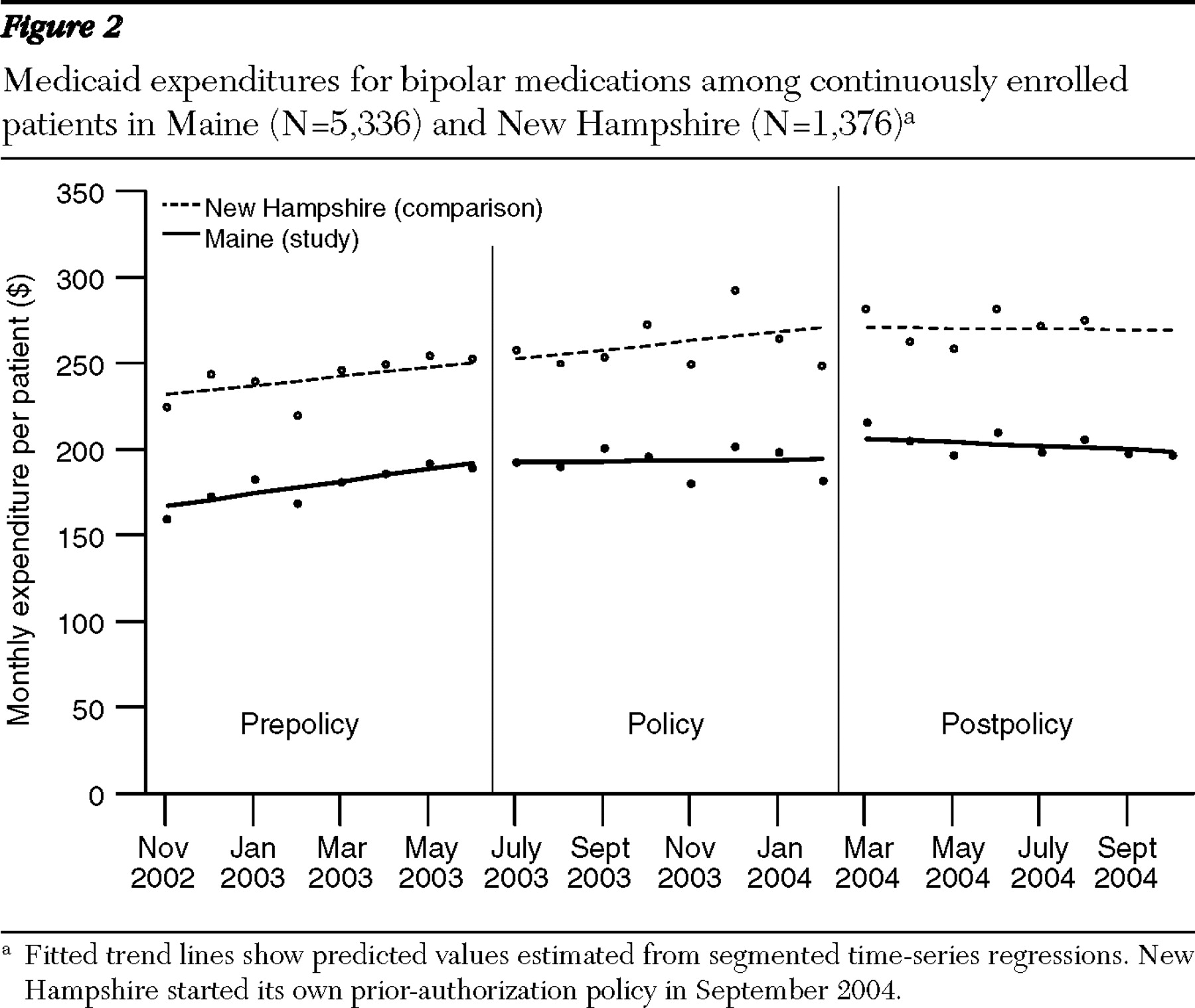
The average monthly costs (adjusted for changes in the consumer price index for health care) of all bipolar medications in Maine was about $167 per patient per month in November 2002, with a monthly increase in trend of $3.60 per patient per month during the eight months before the prior-authorization policy went into effect (
Figure 2 ). In New Hampshire during the same period, the average monthly spending for all bipolar medications was about $232, with a slower monthly increase in trend of $2.80 per patient per month. After the prior-authorization policy was implemented in Maine, the trend leveled off, and in New Hampshire the upward trend continued during the policy period. The prior-authorization policy was associated with a downward trend in pharmacy costs of $3.40 per patient per month. Thus the estimated overall medication savings during the eight-month policy period was about $27 per patient. For 5,336 continuously enrolled patients in Maine, the total savings in drug spending was $144,072. When the prior-authorization policy ended February 29, 2004, the spending trend in Maine was similar to that seen in New Hampshire during the same period.
Changes in rates of bipolar drug treatment discontinuations
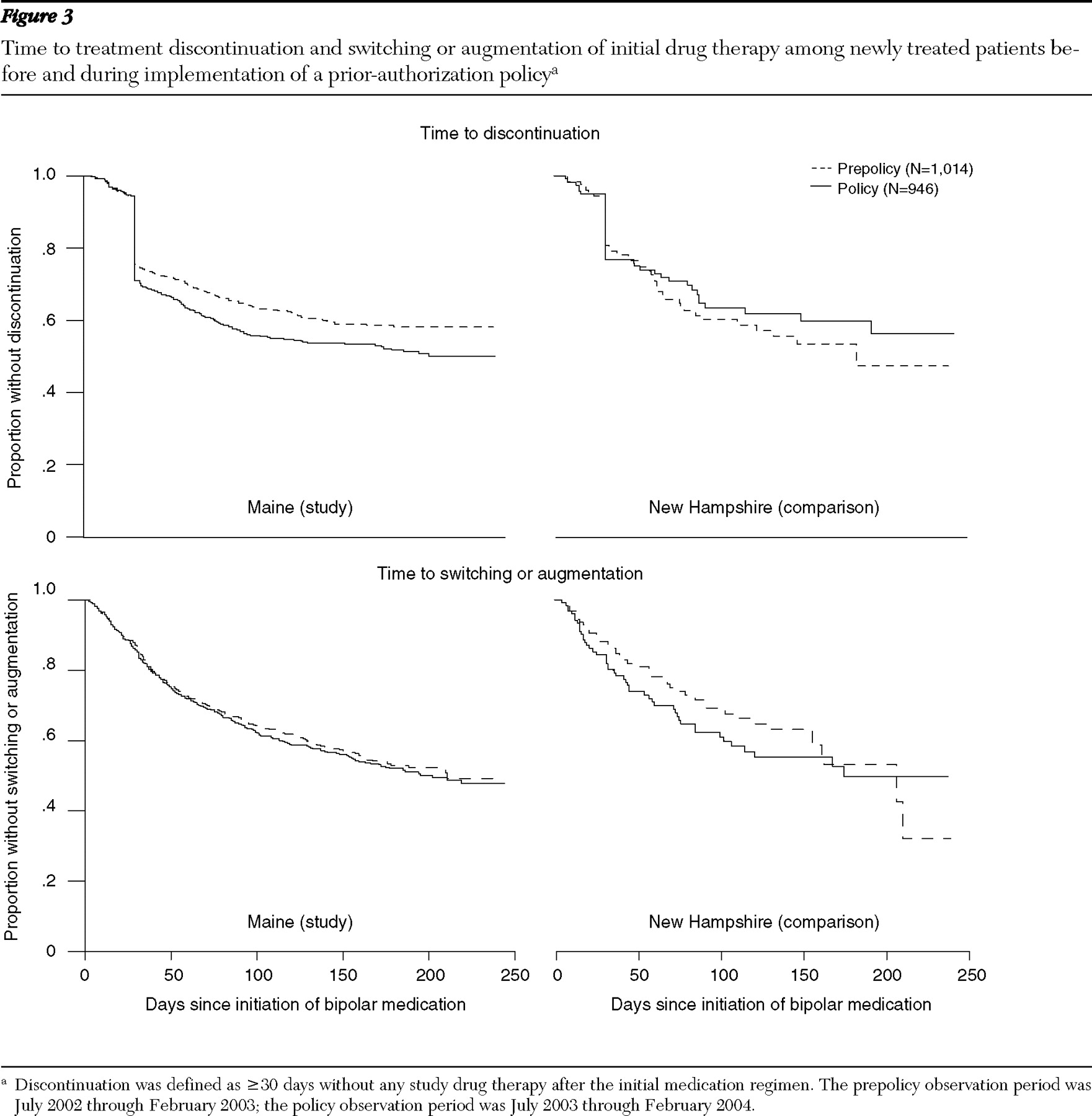
We used Kaplan-Meier survival curves to compare hazard rates for treatment discontinuations between the prepolicy and policy cohorts in the study and comparison states (
Figure 3, top). Compared with the prepolicy period, the policy period was associated with an increase in the proportion of newly treated patients who discontinued therapy. When we controlled for the relative hazard ratios between the prepolicy and policy cohorts in the comparison group, the policy was associated with a 2.28 (95% confidence interval [CI]=1.15–4.52) higher adjusted hazard of discontinuation of all bipolar medications 30 days after therapy initiation, compared with the prepolicy cohort. This effect was not observed for treatment discontinuation within 30 days of initiation (relative hazard rate=.97, 95% CI=.54–1.74). In the policy cohort in Maine, the percentages of patients who discontinued treatment at any given point in follow-up were consistently higher than in the prepolicy cohort, representing an increase in risk. At 30 days after initiation of medication, there was a 5-percentage point absolute increase between policy and prepolicy cohorts in rates of discontinuation in Maine and a 6-percentage point decrease between cohorts in New Hampshire; at 50 days, these differences were a 6-percentage point increase in Maine and a 4-percentage point decrease in New Hampshire; and at 250 days, there was a 10-percentage point increase in Maine and a 6-percentage point decrease in New Hampshire.
Changes in rates of switching and augmentation
There were no differences in hazard rates of switching or augmentation of the initial medication regimen between the policy and prepolicy cohorts observed in both the study and comparison states (
Figure 3, bottom). This finding suggests that the prior-authorization policy did not affect the switching or augmentation rates of the initial regimen of bipolar medication.
Discussion
Psychiatric medications account for 15% of total Medicaid drug spending, and expenditures have increased by 150% from 1998 to 2002 (
3 ). State Medicaid programs face increasing pressure to control drug expenditures and to rely on prior-authorization policies. However, few well-controlled studies have examined the economic and clinical impacts of such policies. This study is the first to use a strong quasi-experimental design to examine the impact of requiring prior authorization for bipolar medications among vulnerable Medicaid enrollees. We found that the Maine prior-authorization policy did not affect the use of preferred drugs but decreased the use of nonpreferred drugs and decreased medication costs by $3.40 per patient with bipolar illness per month during the eight-month policy period. Some of this savings was likely offset by the unmeasured administrative costs of prior authorization itself, previously estimated at approximately $20 per prior-authorization request (
5,
17 ).
Further, under the prior-authorization policy, Maine patients newly treated with nonpreferred medicines were at greater risk of discontinuation of treatment with any bipolar medication compared with similar patients in New Hampshire; this 2.28 (CI=1.15–4.52) increase in adjusted risk of discontinuation mainly occurred between 30 days and 250 days after the initiation of therapy. The absolute difference in risk was smaller, at 30 days and 50 days after initiation. These results are consistent with our previous analyses of prior-authorization effects among patients with schizophrenia, which showed that the policy cohort in Maine had a 1.29 (CI=1.02–1.63) greater hazard of treatment discontinuity than the prepolicy cohort. However, we found no evidence of an increase in medication switching or augmentation with other medications. Individuals with bipolar disorder each respond to drug therapy differently, and therapy often must be customized according to a patient's individual historical response to specific medications or current response to treatment (
18 ). To the extent that prior authorization creates administrative barriers to tailoring medication management for particular patients, their treatment may be discontinued. Thus the preponderance of savings from the Maine policy may be due to discontinuation of therapy rather than to switching to a preferred agent. Discontinuation of all medication treatment is an undesirable outcome, because bipolar disorder is a chronic illness and premature discontinuation of treatment results in higher rates of relapses, hospitalization, and suicide (
12 ).
These findings were highly consistent with our previous study of patients with schizophrenia, which indicated a need to assess the long-term clinical and economic consequences of the increased use of prior-authorization policies in Medicaid and Medicare for drug classes that are likely to differentially affect persons with severe mental illness (
14 ). More information is needed to assess the clinical impacts of these policies across a range of clinical conditions and population subgroups. Prior-authorization policies may be an effective tool to encourage use of appropriate medications. However, special measures may be required to protect the health of vulnerable groups of patients with mental illness (
19 ). One key principle is to update preferred drug lists to incorporate the most recent clinical evidence on safety, effectiveness, and cost-effectiveness of medications. For example, the Agency for Healthcare Research and Quality's Developing Evidence to Inform Decisions About Effectiveness (DEcIDE) network may provide comparative effectiveness data to inform drug policy decisions (
20 ).
There are a few limitations of our study. First, we did not assess the policy's impact on use of medical care. In addition, we could not measure whether patients in either state obtained care in the other state's Medicaid program. Our estimates of changes in drug expenditures postpolicy did not include potential increases in rebates offered to the state Medicaid program by companies whose products were on the preferred list.
Conclusions
Our findings suggest that prior-authorization policies, although reasonable in their purpose to reduce pharmacy spending, might still lead to unintended and unexpected consequences, especially for patients with chronic mental illness. The goal of the Maine prior-authorization program was to direct initial treatment choice toward less expensive medications. If the initial preferred medication did not work well, patients could be switched to second-line treatment. However, rather than resulting in an increase in medication switching, the strongest policy impact was discontinuation of all medication treatment. Until the broad economic and clinical impacts of prior-authorization policies targeting essential psychiatric medications are better known, these policies should be used with caution among vulnerable patients with chronic mental illness.
Acknowledgments and disclosures
This study was mainly funded by grant 63213 from the Robert Wood Johnson Foundation's changes in health care financing and organization initiative. Dr. Y. Zhang acknowledges funding from a Thomas O. Pyle fellowship and a fellowship in pharmaceutical policy at the Department of Ambulatory Care and Prevention, Harvard Medical School (where this work was conducted). Support was also provided by the HMO Research Network Center for Education and Research in Therapeutics (CERT), funded by the Agency for Healthcare Research and Quality (AHRQ).
With the exception of Dr. Y. Zhang, who reports no competing interests, the authors report conducting a previous study of prior authorization of medications for schizophrenia that was supported by a public-private partnership program of AHRQ, CERT, and the HMO Research Network CERT; Eli Lilly and Company; the Centers for Disease Control and Prevention; and the Harvard Pilgrim Health Care Foundation.