Clozapine has been found to be beneficial for a proportion of patients with severe, treatment-resistant schizophrenia. Criteria defining treatment resistance have been developed (
1), and access to treatment with clozapine is often restricted to patients who meet these criteria. However, it is important to establish whether treatment with clozapine could also be beneficial for less severely ill patients who show only a partial response to other antipsychotic drugs.
We were particularly interested in the impact of clozapine treatment on cognitive functioning in this patient group, as cognitive impairment has been shown to be an important determinant of outcome (
2). Some studies have found that clozapine has beneficial effects on cognition (
3,
4), while others have reported detrimental or mixed effects (
5,
6,
7).
Buchanan and associates (
8) reported on a ten-week double-blind study treating partly neuroleptic-responsive patients with schizophrenia with either haloperidol or clozapine. They found that the patients treated with haloperidol did significantly worse on some neuropsychological tests, but neither group showed significant improvement. The patients who took clozapine continued treatment for one year, and during this time their performance improved on some tests. Lee and associates (
9) also compared two groups of non-treatment-resistant patients with schizophrenia who were treated with haloperidol or clozapine. Cognitive functioning improved in both groups, but the group treated with clozapine improved significantly more on the Digit Symbol Test and the Controlled Oral Word Association Test.
In our study, 19 non-treatment-resistant patients with schizophrenia were treated with clozapine for at least 16 weeks. The objective was to evaluate change in psychiatric symptoms, cognitive functioning, and quality of life during treatment with clozapine.
Methods
Nineteen patients—five women and 14 men—with a mean±SD age of 30.6±7.8 years participated in the study. All patients met DSM-III-R criteria for schizophrenia. At the beginning of the study five patients were hospitalized, three lived in psychiatric hostels, six lived with their families, and five lived alone. The mean age at onset of illness was 20.3±4 years, the mean duration of illness was 10.3±5.3 years, and the mean number of hospitalizations was 8.6±6.8. The subjects' mean premorbid IQ, estimated using the National Adult Reading Test, Revised, was 107.6±7. All patients gave informed consent.
Clinical ratings and neuropsychological testing were performed on two occasions—immediately before the patients began taking clozapine and during clozapine treatment.
During the first assessment, four patients were taking risperidone (6 to 8 mg per day). The other patients were taking typical antipsychotic drugs, with a mean daily dose of 760±708.8 mg of chlorpromazine equivalents. Nine patients were taking anticholinergic drugs, three were taking tricyclic antidepressants, four were taking selective serotonin reuptake inhibitor (SSRI) antidepressants, and four were taking clonazepam.
All other antipsychotic drugs were ceased when clozapine was initiated. Each patient's clozapine dose was determined by the patient's clinical response, tolerance of side effects, and serum levels of the medication. At the second assessment, the mean duration of treatment with clozapine was 6.5±2 months, and the mean daily dose was 393.4±181.6 mg. Seven patients were also taking an SSRI, one was taking sodium valproate, and three were taking anticholinergic drugs.
Clinical rating scales included the Positive and Negative Syndrome Scale (PANSS) and the Quality of Life Scale (QLS) (
10). Cognitive functioning was measured using the Wechsler Adult Intelligence Scale, Revised (WAIS-R) Digit Symbol Substitution Test, a test of visual attention and psychomotor speed; the WAIS-R Block Design Test, which evaluates visuospatial construction; the WAIS-R Similarities Test, a test of verbal concept formation; the Controlled Oral Word Association and Category Instance Generation, which test verbal fluency; the Selected Reminding Test and the Consonant Trigram, which test recall memory; and the Wechsler Intelligence Scale for Children, Revised, Mazes Test, which requires executive function.
Different versions of the tests were used where possible for retesting. Scores were adjusted for age and education. This test battery is similar to that used by Hagger and associates (
3), who reported that patients with schizophrenia did not show significant improvement in performance due to practice when these tests were given on separate occasions.
Paired t tests were used to evaluate the significance of change in clinical and quality-of-life measures. A repeated-measures multivariate analysis of variance (MANOVA) was used to test the hypothesis that there was a significant difference in overall neuropsychological test results on the two testing occasions. Paired t tests were used to evaluate the significance of this improvement on specific tests.
Results
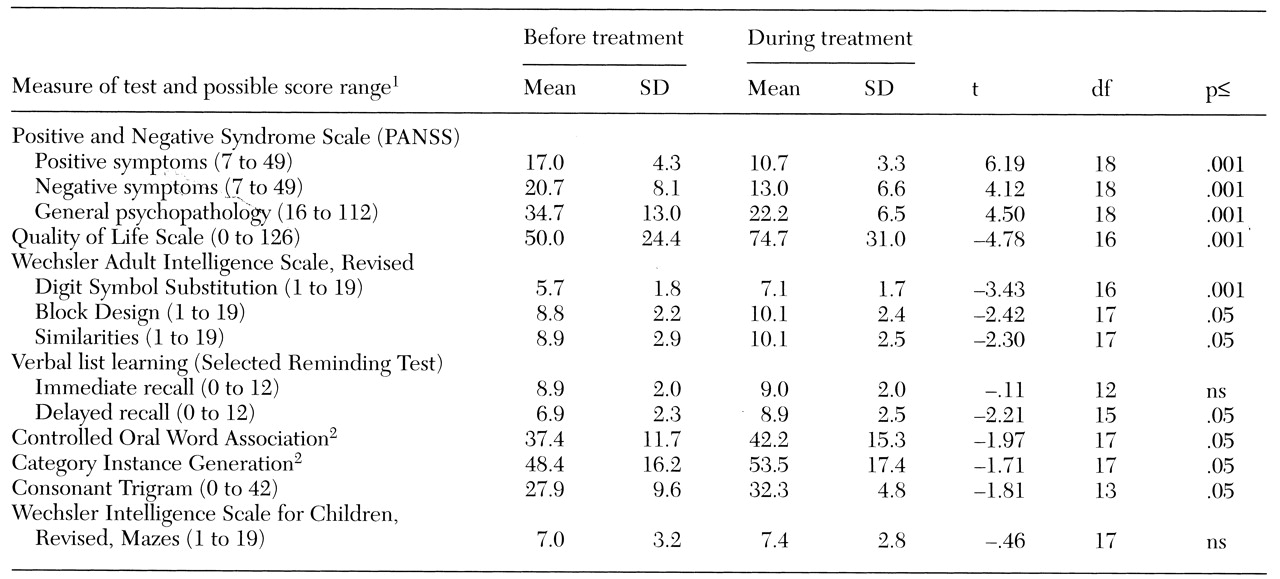
As
Table 1 shows, significant improvement in all measures of psychopathology and the QLS was associated with clozapine treatment. The MANOVA comparing the preclozapine neuropsychological test scores with the scores from the clozapine treatment phase yielded a significant main effect (F=16, df=1,18, p=.001). The results of individual t tests comparing the scores from the two phases are also shown in
Table 1.
Discussion and conclusions
We found that patients who do not meet criteria for nonresponse to typical antipsychotic drugs can benefit from treatment with clozapine. During clozapine treatment the patients in this study experienced a reduction in positive and negative symptoms and an improvement in quality of life. As in previous studies (
3,
4), improvement in cognitive functioning was most evident on tests that require motor speed, such as the Digit Symbol, Controlled Oral Word Association, and Category Instance Generation. It has been suggested that this effect is mediated by a reduction in extrapyramidal symptoms, although Lee and associates (
9) did not find any difference in severity of these symptoms between clozapine-treated patients whose performance on these tests improved and haloperidol-treated patients who showed no change.
The Wechsler Similarities Test measures aspects of conceptual ability. Impaired conceptual functioning, observed clinically as concrete thinking, is common among patients with schizophrenia, and our results suggest that clozapine treatment can produce a modest improvement in this impairment. The Block Design Test is generally recognized as a good measure of visuospatial organization, and again performance was better when the patients were taking clozapine.
We found an improvement in one of two tests of short-term memory and in delayed memory, but interpretation is complicated by the anticholinergic properties of clozapine and the other drugs used. Clozapine treatment has been reported to worsen performance on tests of visual memory and cognitive flexibility (
5,
6,
8), but these functions were not tested in this study.
It appears that clozapine has a range of effects on neuropsychological functioning rather than bringing about global improvement in cognition. However, even on the tests on which patients showed some improvement, performance was still below that expected given the patients' age, education, and premorbid IQ.
This was an open-label study, without a control group, and patients were taking other drugs besides clozapine in both phases of the study. However, our results are consistent with previous studies reporting clinical and cognitive improvement with clozapine treatment among patients who do not meet criteria for treatment-resistant schizophrenia. Treatment with clozapine should therefore be considered for patients who are partly responsive to typical antipsychotic drugs but have persisting residual symptoms and a suboptimal quality of life.