Search Strategies, Study Selection, and Search Results
Literature Searches
Doctor Evidence Original Search Strategy
Search Date: June 7, 2018
| Search ID# | Query | Results |
|---|---|---|
#1 | (“Borderline Personality Disorder”[Mesh]) OR (borderline [tiab] AND personality [tiab]) | 8,962 |
#2 | (“animals”[MeSH Terms] OR animal [tiab] OR animals [tiab] OR rat [tiab] OR rats [tiab] OR mouse [tiab] OR mice [tiab] OR rodent [tiab] OR rodents [tiab]) NOT (“humans”[MeSH Terms] OR humans [tiab] OR human [tiab]) | 4,419,530 |
#3 | #1 NOT #2 | 8,957 |
Limit to English | 7,983 |
| Search | Query | Results |
|---|---|---|
#1 | exp *borderline state/ or (borderline and personality).ti. or (borderline and personality).ab. | 11,073 |
#2 | limit #1 to (article or article in press or conference paper) | 7,571 |
#3 | #2 not ((exp animal/ or nonhuman/) not exp human/) | 7,564 |
#4 | #2 not ((animal or animals or rat or rats or mouse or mice or rodent or rodents) not (humans or human)).ti,ab. | 7,548 |
#5 | #3 or #4 | 7,569 |
#6 | limit #5 to yr = “1883–2002” | 2,765 |
#7 | limit #5 to yr = “2002–Current” | 4,929 |
#8 | remove duplicates from #6 | 2,740 |
#9 | remove duplicates from #7 | 4,739 |
#10 | #8 or #9 | 7,337 |
#11 | limit #10 to English language | 6,356 |
| Search | Query | Results |
|---|---|---|
#1 | MeSH descriptor: [Borderline Personality Disorder] explode all trees | 390 |
#2 | borderline and personality:ti,ab,kw (Word variations have been searched) | 684 |
#3 | #1 or #2 | 684 |
#4 | #3 not (pubmed or embase):an | 145 in trials; 6 in Cochrane reviews; 9 in other reviews |
| Search | Query | Limiters/Expanders | Results |
|---|---|---|---|
S1 | MM “Borderline Personality Disorder” | 5,220 | |
S2 | DE “Borderline Personality Disorder” | 7,857 | |
S3 | MA “borderline personality disorder” | 4,192 | |
S4 | TI “borderline personality” OR AB “borderline personality” OR SU “borderline personality” OR KW “borderline personality” | 11,400 | |
S5 | S1 OR S2 OR S3 OR S4 | 11,400 | |
S6 | (MM “Animals” OR DE “Animals” OR DE “Vertebrates” OR DE “Amphibia” OR DE “Birds” OR DE “Fishes” OR DE “Mammals” OR DE “Pigs” OR DE “Reptiles” OR DE “Rats” OR DE “Rodents” OR DE “Mice”) | 329,022 | |
S7 | TI “animals” OR TI “animal” OR TI “mouse” OR TI “mice” OR TI “rodent” OR TI “rodents” OR TI “rat” OR TI “rats” OR SU “animals” OR SU “animal” OR SU “mouse” OR SU “mice” OR SU “rodent” OR SU “rodents” OR SU “rat” OR SU “rats” OR KW “animals” OR KW “animal” OR KW “mouse” OR KW “mice” OR KW “rodent” OR KW “rodents” OR KW “rat” OR KW “rats” OR AB “animals” OR AB “animal” OR AB “mouse” OR AB “mice” OR AB “rodent” OR AB “rodents” OR AB “rat” OR AB “rats” | 426,155 | |
S8 | Limiters—Population Group: Animal | 385,743 | |
S9 | S6 OR S7 OR S8 | 459,805 | |
S10 | Limiters—Population Group: Human | 3,780,890 | |
S11 | TI “humans” OR TI “human” OR AB “humans” OR AB “human” OR SU “humans” OR SU “human” OR KW “humans” OR KW “human” | 1,585,426 | |
S12 | S10 OR S11 | 3,888,530 | |
S13 | S9 NOT S12 | 310,376 | |
S14 | S5 NOT S13 | 11,398 | |
S15 | Limiters—Publication Type: All Journals | 3,518,961 | |
S16 | S14 AND S15 | 9,386 | |
S17 | LA English | 4,207,720 | |
S18 | S16 AND S17 | 8,116 |
RTI Updated Search Strategy
Search Date: June 15, 2020
| Search | Query | Results |
|---|---|---|
#1 | “Borderline Personality Disorder”[Mesh] OR “Borderline Disorder”[ti] OR “Borderline Personality Disorder”[tiab] OR “borderline-patient”[ti] OR “borderline patient”[ti] OR “borderline-patients”[ti] OR “borderline patients”[ti] | 8,693 |
#2 | #1 AND (“2018/01/01”[Date—Publication]: “3000”[Date—Publication]) | 1,202 |
#3 | #2 AND English[lang] | 1,161 |
| Search | Query | Results |
|---|---|---|
#1 | (‘borderline state’/de OR ‘borderline disorder’:ti OR ‘borderline-patient’:ti OR ‘borderline patient’:ti OR ‘borderline-patients’:ti OR ‘borderline patients’:ti OR ‘borderline personality disorder’:ti,ab,kw) AND [2018-2020]/py AND [english]/lim | 1,777 |
#2 | ‘borderline personality disorder’:ti,kw AND [english]/lim AND [1-1-2018]/sd | 990 |
#3 | #1 OR #2 | 1,924 |
| Search | Query | Results |
|---|---|---|
#1 | (“Borderline Disorder” OR “Borderline Personality Disorder” OR “borderline-patient” OR “borderline patient” OR “borderline-patients” OR “borderline patients”):ti,ab,kw OR [mh “Borderline Personality Disorder”] | 851 |
#2 | #1 with Cochrane Library publication date from Jan 2018 to present, in Cochrane Reviews, Cochrane Protocols, Trials, Clinical Answers, Editorials and Special collections | 412 |
| Search | Query | Results |
|---|---|---|
S1 | if(“Borderline Personality Disorder”) OR mjsub(“Borderline Personality Disorder”) OR mainsubject(“Borderline Personality Disorder”) OR ti(“Borderline Personality Disorder” OR “Borderline Disorder” OR “borderline-patient” OR “borderline patient” OR “borderline-patients” OR “borderline patients”) OR ab(“Borderline Personality Disorder”) Additional limits—Date: After January 01 2018; Language: English | 986 |
Search Date: April 6, 2021
| Search | Query | Results |
|---|---|---|
#1 | “Borderline Personality Disorder”[Mesh] OR “Borderline Disorder*”[ti] OR “Borderline Personality Disorder*”[tiab] OR “borderline patient”[ti] OR “borderline patients”[ti] | 9,260 |
#2 | #1 NOT (“Animals”[Mesh] NOT “Humans”[Mesh]) | 9,258 |
#3 | (#2) AND ((“2020”[Date—Publication]: “3000”[Date—Publication])) Filters: English | 744 |
| Search | Query | Results |
|---|---|---|
S1 | DE “Borderline Personality Disorder” | 8,991 |
S2 | borderline W1 (disorder# OR patient#) | 13,511 |
S3 | S1 OR S2 | 13,511 |
S4 | S3 (Limiters – Publication Year 2020 – 2021; Language: English) | 510 |
Search Date: September 24, 2021
| Search | Query | Results |
|---|---|---|
#1 | “Borderline Personality Disorder”[Mesh] OR “Borderline Disorder*”[ti] OR “Borderline Personality Disorder*”[tiab] OR “borderline patient”[ti] OR “borderline patients”[ti] | 9,488 |
#2 | #1 NOT (“Animals”[Mesh] NOT “Humans”[Mesh]) | 9,486 |
#3 | (#2) AND ((“2020”[Date—Publication]: “3000”[Date—Publication])) Filters: English | 949 |
| Search | Query | Results |
|---|---|---|
S1 | DE “Borderline Personality Disorder” | 9,216 |
S2 | borderline W1 (disorder# OR patient#) | 13,784 |
S3 | S1 OR S2 | 13,784 |
S4 | S3 (Limiters – Publication Year 2020 – 2021; Language: English) | 749 |
Criteria for Inclusion/Exclusion of Studies in the Review
| Criteria | Include | Exclude |
|---|---|---|
Participants/population | Age ≥ 13 | Age < 13 |
Diagnosed with BPD as defined by DSM-IV, DSM-IV-TR, DSM-5 (Section II or Section III), or ICD-10 | Individuals with borderline traits without a specific diagnosis | |
For mixed population studies, BPD must account for ≥ 75% of the total population | Diagnosed with BPD as defined by DSM-III-R | |
Subgroups of interest: | Studies in which the primary research focus is a different diagnosis with co-occurring BPD in a subset (< 75% of the total population) | |
Co-occurring mental disorder | ||
Age | ||
Gender | ||
Race/ethnicity | ||
Genotypes (related to treatment selection, treatment response, or adverse effects) | ||
Intervention(s)/exposure(s) | Yoga | Complementary/alternative treatments not listed for inclusion |
Exercise | Somatic therapies | |
Peer-support interventions | Bioenergetic analysis | |
Psychosocial support | Body psychotherapy | |
Safety planning | Core energetics | |
Service delivery approaches: | Hakomi | |
Stepped-care | Somatic experiencing | |
Collaborative care | Pharmacotherapies | |
Measurement-based care | Acetazolamide | |
Treatment setting comparisons | Ethosuximide | |
Face-to-face sessions | Felbamate | |
Group sessions | Fosphenytoin | |
Online programs | Lacosamide | |
Therapeutic community | Methsuximide | |
Video | Pentobarbital | |
Progressive muscle relaxation | Perampanel | |
Somatic therapies: | Primidone | |
Electroconvulsive therapy (ECT) | Rufinamide | |
Repetitive transcranial magnetic stimulation (rTMS) | Droperidol | |
Transcranial alternating current stimulation (tACS) | Nalmefene | |
Transcranial direct current stimulation (tDCS) | Butabarbital | |
Transcranial magnetic stimulation (TMS) | Secobarbital | |
Pharmacotherapies | ||
Anticonvulsant “mood stabilizers”: | ||
Carbamazepine | ||
Divalproex sodium | ||
Gabapentin | ||
Lamotrigine | ||
Levetiracetam | ||
Oxcarbazepine | ||
Phenytoin | ||
Pregabalin | ||
Tiagabine | ||
Topiramate | ||
Valproate | ||
Valproic acid | ||
Vigabatrin | ||
Zonisamide | ||
Antidepressants: | ||
Amitriptyline | ||
Amoxapine | ||
Bupropion | ||
Citalopram | ||
Clomipramine | ||
Desipramine | ||
Desvenlafaxine | ||
Doxepin | ||
Duloxetine | ||
Escitalopram | ||
Fluoxetine | ||
Fluvoxamine | ||
Imipramine | ||
Isocarboxazid | ||
Maprotiline | ||
Mirtazapine | ||
Milnacipran | ||
Nefazodone | ||
Nortriptyline | ||
Paroxetine | ||
Phenelzine | ||
Protriptyline | ||
Sertraline | ||
Selegiline | ||
Tranylcypromine | ||
Trazodone | ||
Trimipramine | ||
Venlafaxine | ||
Vilazodone | ||
Vortioxetine | ||
Antipsychotics: | ||
Aripiprazole | ||
Asenapine | ||
Chlorpromazine | ||
Clozapine | ||
Fluphenazine | ||
Haloperidol | ||
Iloperidone | ||
Loxapine | ||
Lurasidone | ||
Olanzapine | ||
Paliperidone | ||
Perphenazine | ||
Pimozide | ||
Prochlorperazine | ||
Quetiapine | ||
Risperidone | ||
Thioridazine | ||
Thiothixene | ||
Trifluoperazine | ||
Ziprasidone | ||
Benzodiazepines: | ||
Alprazolam | ||
Clobazam | ||
Clonazepam | ||
Clorazepate | ||
Chlordiazepoxide | ||
Diazepam | ||
Estazolam | ||
Flurazepam | ||
Lorazepam | ||
Midazolam | ||
Oxazepam | ||
Quazepam | ||
Temazepam | ||
Triazolam | ||
Opioid agonists and antagonists: | ||
Buprenorphine | ||
Naloxone | ||
Naltrexone | ||
Sedative-hypnotic medications: | ||
Eszopiclone | ||
Melatonin | ||
Ramelteon | ||
Suvorexant | ||
Tasimelteon | ||
Zaleplon | ||
Zolpidem | ||
Other pharmacotherapies: | ||
Clonidine | ||
Lithium | ||
Prazosin | ||
Psychotherapies: | ||
Acceptance and commitment therapy (ACT) | ||
Client-centered therapy | ||
Cognitive analytic therapy (CAT) | ||
Cognitive-behavioral therapy (CBT) | ||
Cognitive rehabilitation | ||
Cognitive therapy (CT) | ||
Comprehensive validation therapy | ||
Dialectical behavior therapy (DBT) | ||
Dual-focused schema therapy | ||
Dynamic deconstructive psychotherapy (DDP) | ||
Emotion regulation group intervention | ||
Emotion regulation training (ERT) | ||
Good psychiatric management (GPM) | ||
Group analytic psychotherapy | ||
Humanistic and integrative psychotherapy | ||
Individual psychotherapy | ||
Interpersonal group psychotherapy | ||
Interpersonal psychotherapy (IPP) | ||
Interpersonal therapy (IPT) | ||
Manual-assisted cognitive therapy (MACT) | ||
Mentalization-based therapy (MBT) | ||
Mindfulness-based cognitive therapy (MBCT) | ||
Motive-oriented therapeutic relationship (MOTR) | ||
Nidotherapy | ||
Problem-solving therapy | ||
Psychoanalytic therapy (psychoanalysis) | ||
Psychodynamic interpersonal therapy (PIT) | ||
Psychodynamic therapy | ||
Psychodynamic/psychoanalytic psychotherapy | ||
Psychoeducation | ||
Psychotherapy focused on psychic representation | ||
Rogerian supportive therapy | ||
Schema-focused cognitive therapy | ||
Schema-focused therapy | ||
Schema-focused psychotherapy (SFP) | ||
Sequential brief Adlerian psychodynamic psychotherapy | ||
Supervised team management | ||
Supportive therapy | ||
System-based psychotherapy | ||
Systemic therapy | ||
Systems Training for Emotional Predictability and Problem Solving (STEPPS) | ||
Transference-focused psychotherapy (TFP) | ||
Comparator(s)/control | Interventions listed above for inclusion | Interventions listed as excluded above for interventions/exposures |
Placebo | ||
Treatment as usual | ||
Wait-list control | ||
Community treatment by experts | ||
General psychiatric management | ||
Standard group treatment | ||
Standard psychiatric care | ||
Structured clinical management | ||
Outcomes | Pre-specified outcomes and outcome measures | Outcomes not listed, imaging markers, physiological markers, and biomarkers |
A. BPD symptoms/diagnostic criteria | Outcomes that were not pre-specified, e.g., during post-hoc, exploratory analyses | |
1. Frantic efforts to avoid real or imaginary abandonment | ||
2. Pattern of unstable and intense interpersonal relationships characterized by alternating between extremes of idealization and devaluation | ||
a. Inventory of Interpersonal Problems (IIP) | ||
b. Distorted self-image | ||
3. Identity disturbances: markedly and persistent unstable self-image or sense of self | ||
a. Distorted self-image | ||
4. Impulsivity | ||
a. Impulsivity | ||
b. Impulsive/behavioral | ||
c. Risk taking behaviors | ||
d. Lack of restraint | ||
e. Barratt Impulsiveness Scale (BIS-11) | ||
f. Multi-Impulsivity Scale (MIS) | ||
5. Recurrent suicidal behavior, gestures, or threats; or self-mutilating behavior | ||
a. Nonsuicidal self-injury | ||
b. Suicide attempts | ||
c. Suicide | ||
d. Suicidal ideation | ||
e. Self-destructive behavior | ||
f. Beck Scale for Suicide Ideation (BSS) | ||
g. Self-Harm Behavior Survey | ||
h. Suicidal Behaviors Questionnaire (SBQ) and SBQ-R | ||
i. Parasuicide History Interview (PHI) | ||
j. Borderline Personality Disorder Severity Index (BPDSI) Parasuicidality Subscale | ||
k. Columbia Suicide Severity Rating Scale (C-SSRS) | ||
l. Deliberate Self-Harm Inventory (DSHI) | ||
m. Self-Injurious Thoughts and Behaviors Interview-Self-Report | ||
6. Affective instability, due to a marked reactivity of mood | ||
a. Irritability | ||
b. Mood swings | ||
c. Difficulties in Emotion Regulation Scale (DERS) | ||
d. Affective dysregulation | ||
7. Chronic feelings of emptiness | ||
8. Inappropriate intense anger or difficulty controlling anger | ||
a. Aggression | ||
b. Anger | ||
c. Hostility | ||
d. Aggressive behavior | ||
e. Antisocial behavior | ||
f. Spielberger State-Trait Anger Expression Inventory (STAXI) | ||
g. Spielberger State-Trait Anger Scale (STAS) | ||
h. Acting Out Scale (AOS) | ||
i. Aggression Questionnaire (AQ) | ||
j. Anger, Irritability, and Assault Questionnaire (AIAQ) | ||
k. Overt Aggression Scale (OAS) | ||
l. Buss Durkee Hostility Inventory (BDHI) | ||
9. Transient, stress-related paranoid ideation, or severe dissociative symptoms | ||
a. Dissociation | ||
B. Scales for BPD | ||
1. Borderline Personality Disorder Severity Index (BPDSI) | ||
2. Zanarini Rating Scale (ZAN-BPD) | ||
C. Other symptoms commonly found in individuals with BPD, but not part of the diagnostic criteria | ||
1. Depression and Anxiety | ||
a. Spielberger State-Trait Anxiety Inventory (STAI) | ||
b. Symptom Checklist-90 (SCL-90) | ||
c. Beck Anxiety Inventory (BAI) | ||
d. Beck Depression Inventory (BDI) | ||
e. Beck Hopelessness Scale (BHS) | ||
f. Hamilton Rating Scale for Anxiety (Ham-A) | ||
g. Hamilton Rating Scale for Depression (Ham-D) | ||
h. Hospital Anxiety and Depression Scale (HADS) | ||
i. Montgomery-Åsberg Depression Rating Scale (MADRS) | ||
j. Patient Health Questionnaire (PHQ-9) | ||
k. Brief Symptom Inventory (BSI) | ||
l. Generalized Anxiety Disorder 7-item scale (GAD-7) | ||
m. Patient Health Questionnaire–Adolescent | ||
n. Patient Health Questionnaire: Somatic, Anxiety, and Depressive Symptoms | ||
D. Functioning Scales | ||
1. Global Adjustment Scale | ||
2. Global Assessment of Functioning (GAF) | ||
3. Quality of Life | ||
4. Global Social Adjustment (GSA) | ||
5. Global Severity Index (GSI) | ||
6. Number of years with employment | ||
7. Social Adjustment Scale (SAS) | ||
8. Social and Occupational Functioning Assessment Scale | ||
9. Social Functioning Questionnaire (SFQ) | ||
10. Social History Interview (SHI) | ||
11. Social Problem-Solving Inventory | ||
12. World Health Organization—Disability Assessment Schedule (WHO-DAS) | ||
E. Adverse events (AEs) | ||
1. Rate of any AEs | ||
2. Overall serious treatment-related AE rate | ||
3. Specific serious treatment-related AEs | ||
4. Study withdrawal due to AE | ||
5. Study withdrawal for any reason | ||
Timing | Treatment duration ≥ 8 weeks | Treatment duration < 8 weeks |
Setting/context | Very high Human Development Index (HDI) countries* | All other countries |
Study design | RCTs phase 2 | 3 | 4 | Single-arm dose-finding trials |
Nonrandomized clinical trials (N ≥ 50): | Observational, noncomparative | |
Phase 1 | 2 | 3 | 4 | Case reports/series | |
Observational studies, comparative (N ≥50) | Prognostic course/factor studies | |
Cross-sectional | Modeling studies | |
Prospective cohort | Pre-clinical | |
Retrospective cohort | Narrative reviews | |
Nonconcurrent cohort | Systematic reviews/meta-analyses (will be used for hand searches) | |
Case-control | ||
Pooled analyses of controlled studies |
Literature Review, Data Abstraction, and Data Management
Assessment of Risk of Bias of Individual Studies
Data Synthesis
Grading the Certainty of Evidence for Major Comparisons and Outcomes
| Grade | Definition |
|---|---|
High | We are very confident that the estimate of effect lies close to the true effect for this outcome. The body of evidence has few or no deficiencies. We believe that the findings are stable (i.e., another study would not change the conclusions). |
Moderate | We are moderately confident that the estimate of effect lies close to the true effect for this outcome. The body of evidence has some deficiencies. We believe that the findings are likely to be stable, but some doubt remains. |
Low | We have limited confidence that the estimate of effect lies close to the true effect for this outcome. The body of evidence has major or numerous deficiencies (or both). We believe that additional evidence is needed before concluding either that the findings are stable or that the estimate of effect is close to the true effect. |
Very low | We have no evidence, we are unable to estimate an effect, or we have no confidence in the estimate of effect for this outcome. The body of evidence has unacceptable deficiencies, precluding reaching a conclusion. |
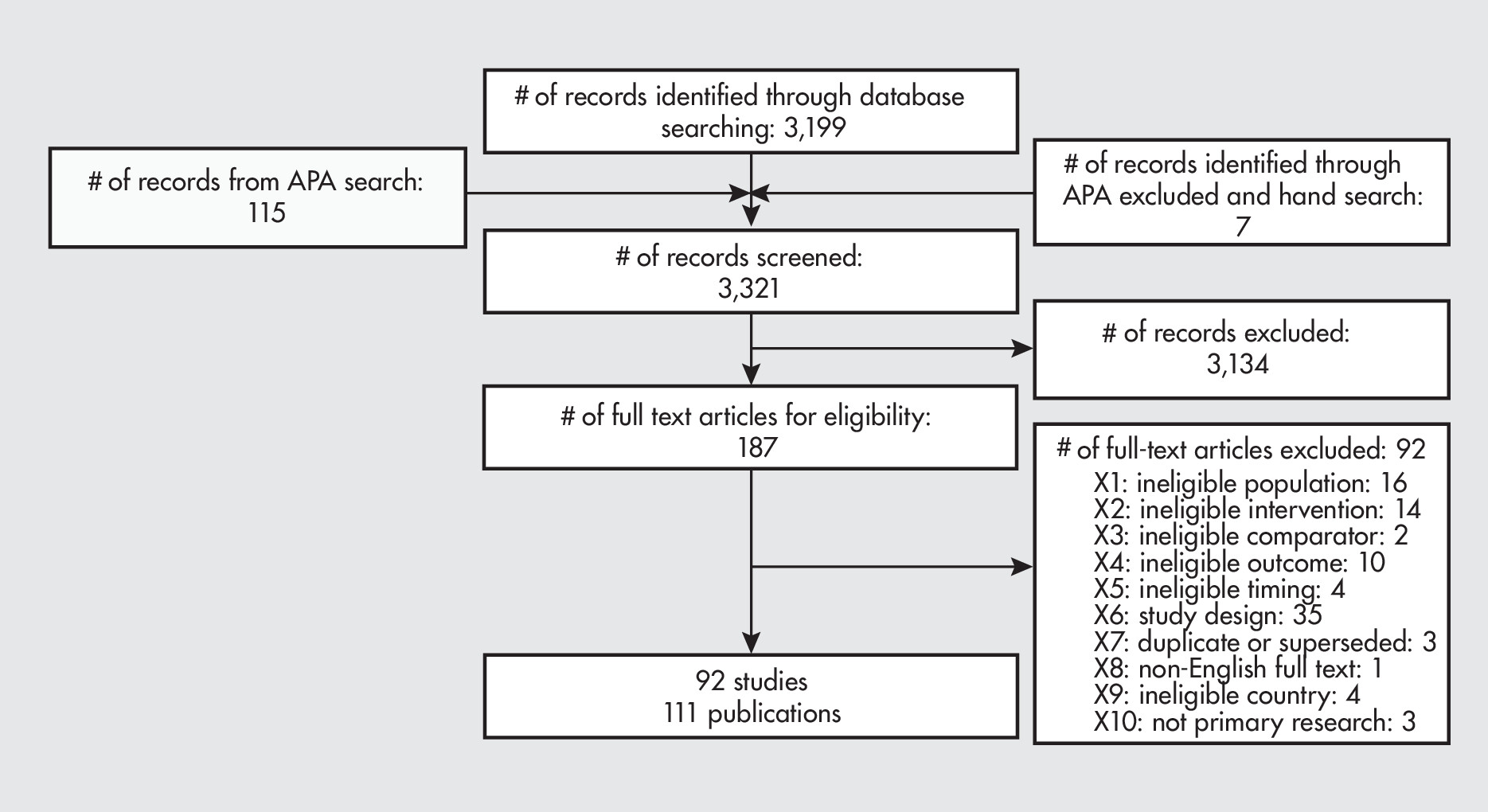
Results of Literature Search and Literature Screening
Information & Authors
Information
Published In
Authors
Metrics & Citations
Metrics
Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
