Although the role of craving in subsequent drug taking continues to be debated, craving is nevertheless an important measurable component of drug abuse whose neurobiological basis remains an enigma (
1). Recently, functional neuroimaging methods have been used to identify brain structures that may mediate cue-induced craving. Using [
18F]fluorodeoxyglucose (FDG) positron emission tomography (PET), Grant et al. (
2) demonstrated metabolic increases in a number of brain regions, including the dorsolateral prefrontal cortex and medial temporal lobes, in cocaine users after exposure to cocaine-related cues. In a preliminary study, Childress et al. (
3) used PET with oxygen-15-labeled water to demonstrate significant increases in blood flow in the amygdala, anterior cingulate, and temporal poles of cocaine users after they viewed videotapes containing cocaine-related cues.
Echo planar functional magnetic resonance imaging (MRI) based on blood oxygenation level dependent contrast is an alternative method for the measurement of brain activation in response to stimuli, providing high spatial and temporal resolution without radioactive tracers (
4,
5). Unlike kinetic parameters estimated with PET, the blood oxygenation level dependent signal does not have a direct physiological significance but, rather, is related to changes in local blood flow and deoxyhemoglobin levels subsequent to neuronal activation (
4,
5). In this study, we evaluated whether cocaine cue-induced regional cerebral activation previously detected with PET (
2,
3) is also detectable with blood oxygenation level dependent functional MRI.
METHOD
Six male subjects (mean age=37 years, SD=7) with a history of crack cocaine use at least once every 2 weeks during the past 6 months and six comparison male subjects (mean age=32 years, SD=4) were studied. Subjects in the cocaine group reported that crack cocaine was their preferred drug of abuse, but a history of other drug use was not grounds for exclusion. Comparison subjects reported no history of cocaine use or drug abuse of any kind. All subjects were screened with the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (
6). Individuals with a history of psychotic illness or a current episode of an axis I mood disorder were excluded. All subjects were in good general health and had no known history of neurological disorder. The protocol was approved by the McLean Hospital Institutional Review Board. All subjects provided written informed consent.
Using segments of the videotape described by Childress and colleagues (
3), we constructed a single 10-minute videotape consisting of four contiguous 150-second segments of alternating neutral and cocaine-related scenes and sounds. This tape was presented to each subject by means of a nonferrous audiovisual system during a single functional MRI scanning session (1.5 Tesla, 7-mm thickness, 3-mm skip, gradient echo, TE=40 msec, TR=5 seconds, α=90°, in-plane resolution=3.125 × 3.125 mm), wherein 120 echo planar images were collected at 5-second intervals in each of 16 coronal slices. To quantify each subject's level of craving, a standardized visual analog scale questionnaire on the subject's strength and frequency of desire for cocaine (
7) was administered twice, immediately before and after scanning. To reduce subject motion artifacts, all functional MRI data were spatially registered and residual trends removed before analysis (
8).
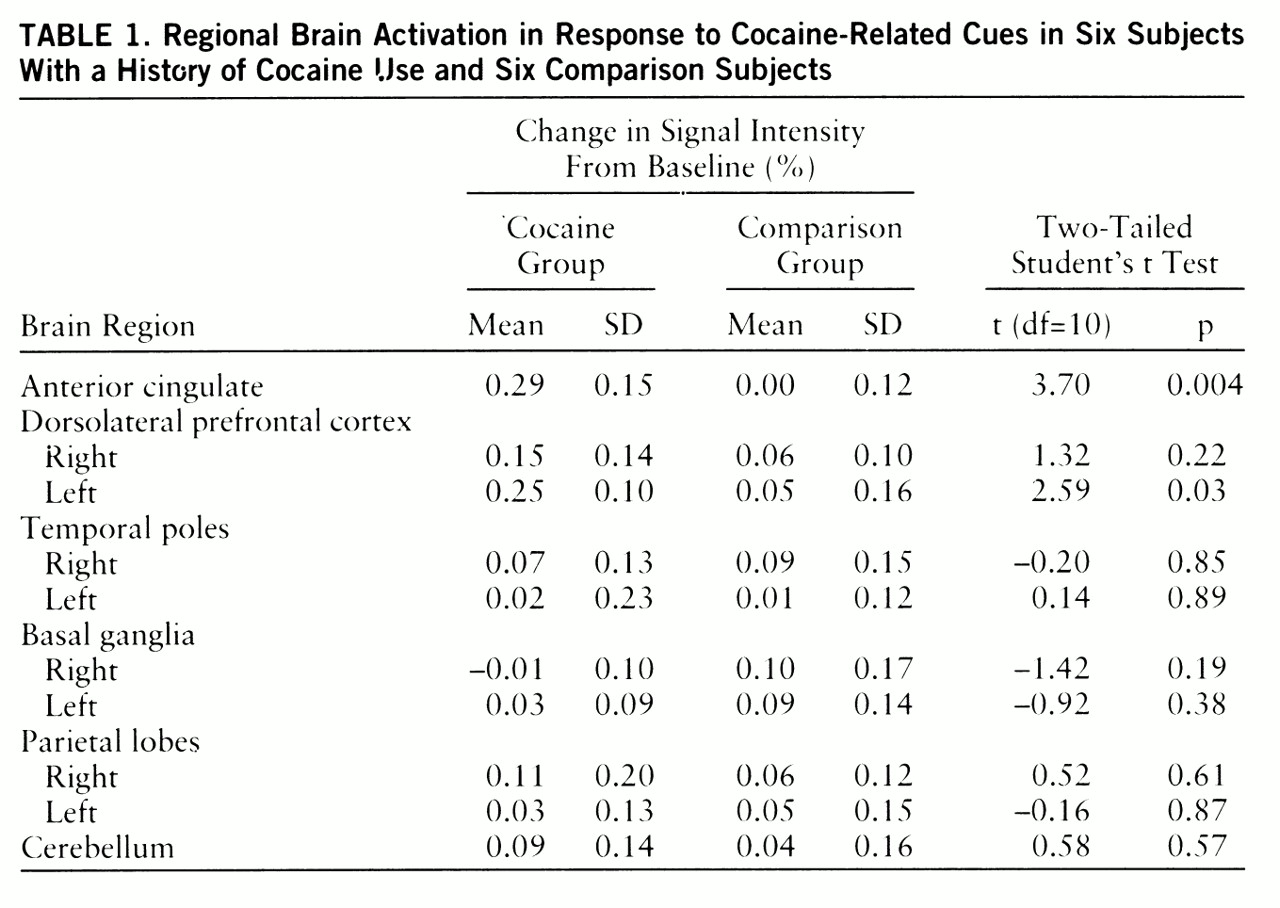
Ten neuroanatomic regions (
table 1) were selected on the basis of the results of prior PET studies (
2,
3). The regions were identified from high-resolution MR images and mapped to the functional image set by two raters. Two scalar measures were used to assess blood oxygenation level dependent activation in each region: 1) the magnitude of activation was defined as the percent change in the mean regional signal intensities measured between the neutral and cocaine-cue segments of the acquisition, and 2) the spatial extent of activation was defined as the fraction of pixels in each region that exceeded an arbitrary activation threshold of r=0.30 according to the correlation coefficient detection technique of Bandettini et al. (
9).
To assess the reliability of the region selection technique, the intraclass correlation was determined from four subjects for both activation magnitude (r1=0.87) and spatial extent (r1=0.84).
RESULTS
Significant differences in activation magnitude between the cocaine and comparison groups were detected in the anterior cingulate and left dorsolateral prefrontal cortex (
table 1). For the cocaine group, the regional signal intensity time courses were comparable for the first and second presentations of each type of stimulus (data not shown), indicating that there were minimal activation carryover effects.
Although significant group differences in spatial extent were not found, spatial extent, but not activation magnitude, correlated significantly with the change in self-reported desire for cocaine in the cocaine group (anterior cingulate: r=0.88, t=3.67, p=0.02; left dorsolateral prefrontal cortex: r=0.83, t=2.97, p=0.04; linear regression, df=4). Because the craving questionnaire was administered only twice, segment-to-segment and carryover effects on craving were not assessed in this study. Significant prescan to postscan changes in craving score, however, were observed only in the cocaine group (mean change=2.83, SD=3.31) (t=2.96, df=11, p<0.03, two-tailed paired t test).
DISCUSSION
We evaluated regional cerebral activation in response to cocaine-related cues by using echo planar functional MRI. Significant activation was detected in the anterior cingulate and left dorsolateral prefrontal cortex of crack cocaine smokers. These findings are consistent with the metabolic (
2) and blood flow (
3) changes found in prior PET studies. As in the FDG PET study (
2), we found that the change in the self-reported level of cocaine craving correlated with a measure of activation in these regions. Susceptibility artifacts near air-tissue interfaces limited consistent imaging of the medial aspect of the temporal lobe in the present investigation and may also have contributed to the negative findings in the temporal poles.
There are a number of procedural differences that may also contribute to the slightly different activation profile observed in the present study. First, in the PET studies (
2,
3), subjects were exposed to cocaine-related cues for 30 minutes. In contrast, we exposed subjects to the stimuli for only 150 seconds at a time. Second, prior studies have imaged only one condition (i.e., drug-related or neutral) per session, allowing subjects at least 1 hour (
3) or 1 week (
2) between conditions. In contrast, we sought to determine if activation could be observed within a single 10-minute session by alternating relatively brief segments of neutral and cocaine-related cues. The results suggest that functional MRI can detect activation during this short period without substantial carryover effects. Third, prior study designs have also included tactile stimuli or the anticipation of cocaine use to increase the likelihood of eliciting craving (
2). In the present study, significant activation and craving were detected by using only visual and auditory cues. We believe that these procedural differences represent a strength of this study in that significant patterns of activation were observed during brief periods of stimulation encompassing only two sensory modalities.
Using functional MRI techniques, we demonstrated that the presentation of cocaine cues activated anterior cingulate and dorsolateral prefrontal cortex, consistent with previous PET results. These results suggest that functional MRI may be a valuable tool in the identification of the neurobiological basis of craving and in the evaluation of new agents to modify or reduce craving (
10,
11).