Given the increasing importance of substance abuse among women, we know little about the sources of individual differences in the vulnerability to cannabis use and abuse. Several lines of evidence, however, suggest that genetic factors might be important. Prior family studies, which have included only a small number of drug-abusing female probands (e.g., references 7 and 8), have suggested that substance abuse by women “runs” in families. Twin and adoption studies of women suggest that genetic factors contribute to the risk of use and abuse of the licit psychoactive substances nicotine (
9–
11) and ethanol (
12,
13). The risk of substance abuse by female adoptees is increased by the presence of alcohol problems and/or antisocial personality in their biological parents (
14,
15). We are aware of only one large-scale epidemiologic twin study of cannabis abuse, conducted with male U.S. veterans, which found evidence for both significant genetic and significant familial-environmental effects on the liability to marijuana abuse (
16). We are unaware of any prior study that has specifically examined cannabis use or misuse in a genetically informative population of women.
METHOD
Our data are derived from a study of genetic and environmental risk factors for common psychiatric and substance use disorders in Caucasian female same-sex twin pairs from the Virginia Twin Registry (
17), a population-based register formed from a systematic review of all birth certificates in the Commonwealth of Virginia. Twins were initially ascertained through mailed surveys to female twin pairs in the Registry, the response to which was approximately 64%. The true cooperation rate was undoubtedly higher, since such large-scale mailings always contain a substantial percentage of questionnaires that do not reach the intended recipients. Twins were then interviewed face-to-face, at which time our refusal rate was about 18%. In the current phase of the project, 2,288 members of female-female pairs from the Virginia Twin Registry were eligible to participate in a structured telephone interview. These twins were unselected except that they had participated in previous face-to-face interviews in this project. Of these 2,288 twins, 1,937 were successfully interviewed in 1995–1997, three had died, 33 were lost to follow-up, one was too medically ill to be interviewed, three had incomplete interviews, 58 neither refused participation nor completed an interview by the end of the study, and 253 refused. Thus, we succeeded in interviewing 84.7% of the entire sample and 86.2% of the eligible sample. Zygosity was determined blindly by standard questions (
18), photographs, and, when necessary, DNA (
17,
19). All interviews were conducted by interviewers blind to information about the co-twin.
This project was approved by the Committee for the Conduct of Human Research at Virginia Commonwealth University. Written informed consent was obtained before the face-to-face interviews and verbal assent before the phone interviews. Lifetime cannabis use, abuse, and dependence were assessed with use of an adaptation of the Structured Clinical Interview for DSM-III-R (
20). The interviewers, each of whom had at least a master's degree in social work or another mental-health-related discipline or a bachelor's degree in a similar area and 2 years of clinical experience, were initially trained for 40 hours and received regularly scheduled review sessions over the course of the study.
Cannabis abuse and dependence were diagnosed according to the DSM-IV criteria. Lifetime heavy use was defined as ever using cannabis on more than 10 occasions in a single month. Three twins had incomplete data on cannabis use, so they were excluded from these analyses. To test the validity of the equal environment assumption—that the exposure of monozygotic and dizygotic twins to environmental risk factors for cannabis use and misuse was equally correlated—we assessed twin similarity for three kinds of environmental exposure: in childhood (how often the twins shared the same room at home and class at school and were dressed alike), in adolescence (how often they had the same friends, were in the same social group, and went together to movies and dances), and in adulthood (how often, in the past year, they were in contact with each other).
We present three different statistics that assess the degree of twins' resemblance in cannabis use, abuse, and dependence.
Probandwise concordance, the most traditional summary statistic for twin studies, is defined as the proportion of co-twins of proband twins who are themselves affected. The
odds ratio reflects the increase in risk for a given trait (i.e., cannabis use, abuse, or dependence) in co-twins of twins with that trait over the risk seen in a randomly selected member of the twin's zygosity group. Odds ratios and their 95% confidence intervals were obtained from the logistic regression procedure in SAS (
21). The
tetrachoric correlation, or correlation of liability (
22,
23), assumes that underlying the observed division of twins into those with and without cannabis use or abuse, there exists a latent distribution that reflects their underlying “liability.” We assume that a threshold exists on this liability distribution such that individuals with a liability above the threshold use cannabis or develop cannabis abuse, while those with a liability below the threshold remain free of such problems. The tetrachoric correlation represents the correlation between members of a twin pair for this underlying liability. This model further assumes that cannabis use, abuse, and dependence have a multifactorial etiology involving a number of genetic and environment risk factors of small to moderate effect (
24). Under these circumstances, the distribution of these liabilities in the general population will be approximately normal.
On the basis of this liability-threshold model, we also perform biometric model fitting to these twin data (for further details, see references 25 and 26). In the full twin model used in this report, resemblance between twins is assumed to result from two sets of latent factors: 1) additive genes (A), which contribute twice as much to the correlation in monozygotic twins as in dizygotic twins (because monozygotic twins share all their genes identical by descent, while dizygotic twins, like nontwin siblings, share on average half of their genes), and 2) family or “common” environment (C), which contributes equally to the correlation in monozygotic and dizygotic twins. In addition to common environment (those environmental factors, such as parental religiosity, that make members of a twin pair similar in liability to cannabis use or abuse), the model also contains individual-specific environment (E), which, in addition to measurement error, is a measure of the impact of the environmental experiences that may make members of a twin pair different in liability to cannabis use or misuse.
The formal analysis of our twin data begins with fitting an ACE model. This model, and all other model fitting reported here, was fitted directly to contingency tables with use of the program Mx (
27) by weighted least squares. The ACE model includes additive genes (A), common environment (C), and individual-specific environment (E). We then fit two simpler models. One of these, the AE model, contains only additive genes (A) and individual-specific environment (E) and assumes that all familial aggregation results from additive genetic effects. The other, the CE model, contains only common environment (C) and individual-specific environment (E) and assumes that all observed familial aggregation is the result of shared environmental influences.
The goal in model fitting is to explain the observed data as well as possible with as few parameters as possible. We operationalize this goal with the use of Akaike's information criterion (
28,
29), which equals the chi-square value minus twice the degrees of freedom. The model with the lowest value of Akaike's information criterion reflects the best balance of goodness of fit and parsimony. In addition, the fit of the CE or AE model can be directly compared with that of the ACE model by a chi-square difference test with one degree of freedom.
The final step in twin analysis is to estimate, on the basis of the best-fitting model, the proportion of variance in liability to cannabis use or misuse that is due to individual-specific environment (e2) and, depending on the results of model fitting, additive gene action (a2) or common environment (c2). The proportion of variance in liability due to additive genetic effects is often termed “heritability.”
Twin studies provide a method for detecting cooperation bias. Since cannabis use is correlated in twin pairs, if cannabis use predicts lack of cooperation, then the rates of cannabis use should be higher among twins whose co-twin refused to be interviewed than among twins whose co-twin was successfully interviewed.
RESULTS
Our sample for analysis contained 1,934 individual twins, including both members of 485 monozygotic pairs and of 335 dizygotic pairs. The mean age of the subjects at interview was 36.6 years (SD=8.1, range=22–62). The mean age at first use of cannabis was 18.8 years (SD=4.7), and the mean age at heaviest use was 20.0 years (SD=4.9). The prevalences of lifetime cannabis use, heavy use, abuse, and dependence in the group were 47.9% (SE=1.1%), 6.7% (SE=0.6%), 7.2% (SE=0.6%), and 2.2% (SE=0.3%), respectively. During the period of maximum intake, the median and mean numbers of times per month that cannabis was used by heavy users were 30 and 41.6, respectively. Of the 927 twins who stated that they used cannabis, 926 (99.9%) reported using marijuana, 243 (26.2%) hashish, and 27 (2.9%) tetrahydrocannabinol.
Possible Biases and Reliability
If the co-twin refused to be interviewed, her twin had a nonsignificantly increased risk of cannabis use (odds ratio=1.32, χ2=3.44, df=1, p=0.06), heavy use (odds ratio=1.29, χ2=0.62, df=1, p=0.43), and abuse (odds ratio=1.34, χ2=0.86, df=1, p=0.35) but a decreased risk of cannabis dependence (odds ratio=0.67, χ2=0.77, df=1, p=0.38). While we found no significant differences between monozygotic and dizygotic twins in the prevalence of heavy use of cannabis (χ2=1.34, df=1, p=0.25), cannabis abuse (χ2=0.03, df=1, p=0.87), or cannabis dependence (χ2=1.58, df=1, p=0.21), dizygotic twins did report a significantly higher rate of cannabis use than monozygotic twins (χ2=7.46, df=1, p=0.01). This difference, however, was small in size (52.8% versus 46.0%, ϕ=0.07).
We tested for the equal environment assumption by attempting to predict, controlling for zygosity, twin pair resemblance in cannabis use, heavy use, abuse, and dependence from the similarity of the twin pair's childhood, adolescent, and adult environments. Of these 12 analyses (results available on request), one had significant results at the 5% level; similarity of adolescent environment significantly predicted twins' similarity in cannabis use (χ2=9.73, df=1, p=0.002).
One hundred ninety-two twins were interviewed twice, by different interviewers; the mean interval was 4.3 weeks (SD=1.5). Chance-corrected interviewer agreement (kappa) (
30) and tetrachoric correlations (r) were as follows: for cannabis use, kappa=0.99, r=0.99; for heavy use of cannabis, kappa=0.65, r=0.91; and for cannabis abuse, kappa=0.67, r=0.91. Cannabis dependence was too rare in this subsample to obtain meaningful reliability estimates.
Twin Concordance Rates and Odds Ratios
As shown in
table 1, probandwise concordance rates for cannabis use in monozygotic and dizygotic pairs were 75% (SE=2%) and 70% (SE=2%), respectively. Of note, the base rate for cannabis use was lower in monozygotic twins than in dizygotic twins, and a significantly higher proportion of monozygotic pairs than dizygotic pairs were concordant for never having used cannabis (42.5%, N=206 of 485, and 31.3%, N=105 of 335, respectively; χ
2=10.4, df=1, p=0.001). Thus, the odds ratio and 95% confidence interval were over twice as great in monozygotic twins as in dizygotic twins.
Probandwise concordances for heavy cannabis use in monozygotic and dizygotic twin pairs were 49% (SE=6%) and 27% (SE=8%), respectively. For cannabis abuse, probandwise concordances were 47% (SE=6%) in monozygotic twins and 16% (SE=5%) in dizygotic twins. The sample contained only two pairs (one monozygotic and one dizygotic) concordant for cannabis dependence, too small a number to obtain stable estimates. Therefore, for model fitting, we examined dependence as a semicontinuous trait, categorizing all twins as meeting none, one to two, or three or more of the DSM-IV criteria for cannabis dependence. Multiple categories permit a test of the liability-threshold model (
31), which fitted well both in monozygotic twins (χ
2=6.0, df=3, p=0.11) and dizygotic twins (χ
2=1.5, df=3, p=0.67).
Twin Model Fitting
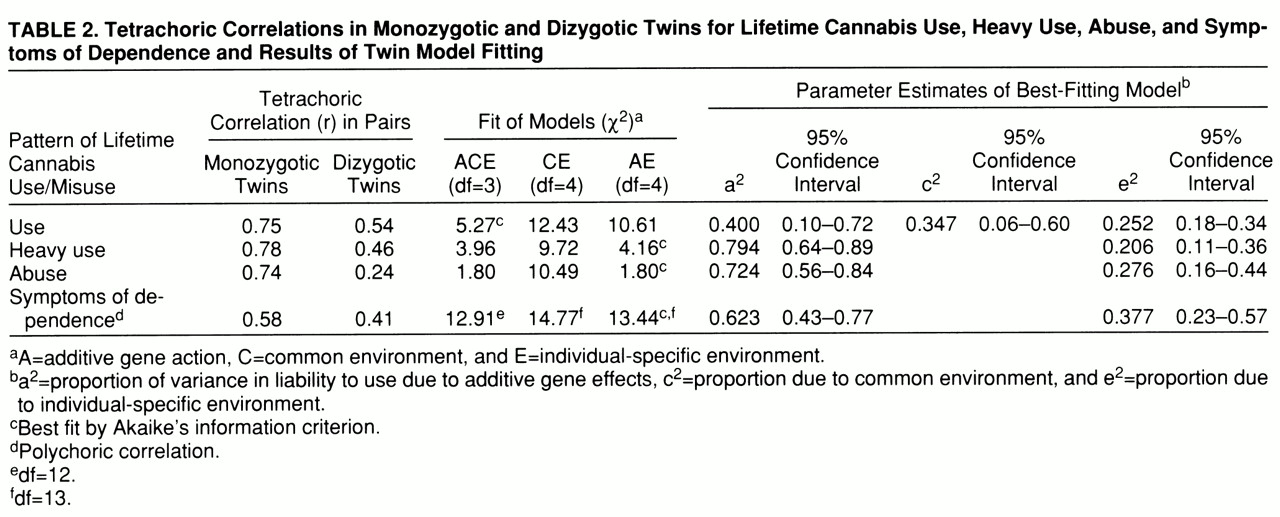
Tetrachoric correlations and the results of twin model fitting are presented in
table 2. The full or ACE model fitted well for cannabis use, heavy use, abuse, and symptoms of dependence. For cannabis use, the model with the best Akaike's information criterion value was the ACE model. Both the CE model (which assumes that twins' resemblance can be explained solely by familial-environmental factors) and the AE model (which assumes that twins' resemblance is due solely to genetic factors) could be rejected against the ACE model by the chi-square test (χ
2=6.8, df=1, p=0.01, and χ
2=5.7, df=1, p=0.02, respectively). The results of the ACE model suggested that twins' resemblance was due both to genetic factors (accounting for 40% of the variance) and to family environment (accounting for 35% of the variance).
For heavy use of cannabis, the AE model had the best Akaike's information criterion value and could not be rejected against the ACE model (χ2=0.2, df=1, n.s.). However, we could reject the CE model, which postulated that twins' resemblance in heavy cannabis use was due solely to familial-environmental influences (χ2=5.8, df=1, p=0.02). The AE model estimated the heritability of liability to heavy cannabis use at 79%.
For cannabis abuse, the AE model also had the best Akaike's information criterion value and exactly the same fit as the more complex ACE model. Again, the CE model could be rejected with a high degree of significance (χ2=8.7, df=1, p=0.003). The heritability of liability to cannabis abuse was estimated in the AE model to be equal to 72%.
In the examination of symptoms of cannabis dependence, the AE model again had the best Akaike's information criterion value, but this was only modestly superior to that found for the ACE model. The ACE model suggested that twins' resemblance in symptoms of cannabis dependence was due both to genetic factors (a2=0.43) and to family environment (c2=0.18). The AE model estimated the heritability of cannabis dependence at 62%.
DISCUSSION
Our results suggest that genetic factors have a substantial impact on the liability of women to develop cannabis use, abuse, and dependence. These findings are consistent with a range of findings that the risks for licit (
9–
13) and illicit (
14,
15) psychoactive drug use by women are influenced by genetic factors. The estimated heritability of cannabis use was moderate and suggested that genetic risk factors accounted for less than half of the overall liability. By contrast, our results suggested that heavy cannabis use, cannabis abuse, and cannabis dependence are highly heritable—that genetic factors are responsible for 60%–80% of the variance in liability. We are aware of only one result in males with which our findings are directly comparable; it was obtained in 3,372 veteran male-male twin pairs (
16). Heritability of marijuana abuse in that sample, defined by DSM-III-R criteria, was 33%, which is below the lower limit of the 95% confidence interval of the estimate obtained in our study. These findings suggest that genetic factors are at least as important in the etiology of cannabis abuse among women as among men.
In contrast to our results for heavy cannabis use, abuse, and dependence—where the best-fitting model indicated that twins' resemblance could be best explained solely by genetic factors—for cannabis use, family environment also appeared to play an important etiologic role. These findings are consistent with prior research which has suggested that familial-environmental factors, such as level of family attachment and parental monitoring, predict adolescent marijuana use (
32,
33). Our results are consistent with the hypothesis that familial-environmental factors influence the probability of initiation of cannabis use. However, given initiation, the risk for progression to heavy use, abuse, and dependence appears to be largely independent of the family environment and heavily determined by genotype.
Of the 12 tests we performed to examine the validity of the equal environment assumption in this study, one produced significant results: the frequency of social contact of the twins as teenagers predicted concordance for marijuana use. While this single finding may be due to chance (
34), the significance level observed suggests that the effect may be real.
Monozygotic twins in this sample reported socializing together as adolescents substantially more frequently than dizygotic twins (t=13.08, df=1371, p<0.0001). If what we term “co-socialization” produces resemblance in cannabis use, this could inflate our estimates of heritability. However, before such a conclusion can be reached, we need to understand why monozygotic twins have higher levels of co-socialization. Two hypotheses are plausible. First, monozygotic twins might socialize together more frequently than dizygotic twins because of social expectations. That is, they and their friends might believe that monozygotic twins should “hang out” together. Second, monozygotic twins might spend a great deal of time together because of genetic factors, since individuals who are genetically alike tend to seek out similar environments (e.g., are inclined to enjoy the same friends and similar social activities). Only if social expectations predominate as the cause of high levels of co-socialization in monozygotic twins would we have reason to be concerned about a violation of the equal environment assumption.
We evaluated these two hypotheses by examining twins' “social” zygosity (what kind of twin they believe themselves to be) and their “true” zygosity (assigned by us on the basis of standard questions, photographs, and DNA) (
35). In about 10% of the twins, their social and true zygosities differed. We predicted, in a stepwise linear regression, the twins' co-socialization scores from both their true and social zygosities. True zygosity was entered first and accounted for 10.1% of the variance (F=40.60, df=1,1767, p<0.0001). Social zygosity was entered second and also significantly predicted co-socialization scores (F=4.59, df=1,1767, p=0.03) but accounted for only 0.2% of the variance. The results may be more clearly seen from the mean standardized co-socialization scores for the four twin types (with higher scores indicating more frequent social contact with the co-twin): true monozygotic/social monozygotic (N=875 twins), score=0.30; true monozygotic/social dizygotic (N=147), score=0.15; true dizygotic/social monozygotic (N=22), score=–0.11; true dizygotic/social dizygotic (N=726), score=–0.37.
These results support the second hypothesis more than the first. Monozygotic twins tend to have the same friends and go to the same events because, being genetically identical, they prefer the same kind of social activities. Social expectations, as indexed by their social zygosity, play only a minor role in influencing the frequency of co-socialization in adolescent twins.
What, then, is the true heritability of cannabis use? Currently, we only possess the ability to correct heritability by assuming the first hypothesis (
35). Under this “worst-case” scenario—that social factors are completely responsible for the association between zygosity and frequency of social contact in adolescence—the heritability of cannabis use declined from 40% to 17%. This correction, however, is overly conservative. If we estimate that about 80% of the excess levels of co-socialization among monozygotic versus dizygotic twins are genetic and about 20% are due to the social environment, then we can calculate that the true heritability of cannabis use is about 35%, slightly lower than the rate originally estimated.
These analyses suggest that genetic factors are likely to contribute to cannabis use in part by influencing the probability of an individual's placing herself in a social environment in which cannabis use is encouraged. This phenomenon, which we have called “genetic control of exposure to the environment” (
36), is likely to be widespread (
37). Prior analyses in this sample suggest that genetic factors influence an individual's risk of experiencing stressful life events (
38) and low levels of social support (
39).
Consistent with most prior studies (
40,
41), our test-retest results suggested that we were able to assess cannabis use, abuse, and dependence with relatively good reliability. In standard twin studies, errors of measurement result in an overestimation of the individual-specific environment (e
2) and an underestimation of heritability (a
2) (
26). The good reliability of our assessments of cannabis-related behaviors suggests that these biases in parameter estimation are likely to be modest.
The proportion of our sample that reported ever using cannabis (47.9%) is slightly higher than that found for U.S. women in the National Comorbidity Survey (41.0%) (
1) and for U.S. white women above the age of 17 in the 1993 National Household Survey on Drug Abuse (46.4%) (
2). The lifetime prevalence of DSM-IV cannabis dependence in our sample (2.2%) is almost identical to the estimate of 2.3% for U.S. women, based on DSM-III-R criteria, reported in the National Comorbidity Survey (
1). These results suggest that our findings in female twins from the Virginia Twin Registry are likely to be broadly representative of white adult women across the United States.
These analyses do not address the critical question of the nature of the pathway from genes to cannabis use and abuse. Many factors are probably involved, including those that increase the risk of use of a range of substances—such as personality (
15,
42) and liability to psychiatric syndromes including conduct disorder, attention deficit hyperactivity syndrome, and antisocial personality (
43–
45)—and those that may be more specific to cannabis, including variation in cannabinoid metabolism, distribution (
4), and interaction with target receptors (
46). Among individuals who experiment with marijuana, the hedonic quality of the intoxication predicts future use (
47), and the liability to experience positive and negative effects from marijuana use may be influenced by genetic factors (
48).
The results of this study should be interpreted in the light of four potential methodologic limitations. First, although our total sample of twins was large, the number of twins with cannabis abuse and dependence was small. While we were able to establish the etiologic role of genetic factors in cannabis abuse and dependence, the group of available subjects was insufficient to determine, with high precision, the magnitude of the genetic effect.
Second, although our sample was derived from a population-based twin registry, our final study group was unlikely to be completely representative of the entire twin population. Twins who did not participate at earlier stages of our research were not included in this sample. While we found no significant relationship between cannabis use and misuse in one twin and the success of interviewing her co-twin, three of four of the nonsignificant trends were in the same direction; lack of cooperation of the co-twin was associated with the use and abuse of cannabis by her twin. These results are consistent with most studies (
49,
50) in suggesting that individuals with substance abuse may be overrepresented among individuals who refuse to cooperate in general population surveys. Given that the prevalence of cannabis use in our sample was similar to that obtained in other large national surveys, it seems unlikely that unusual biases with respect to drug use have entered into the composition and subsequent study of our population-based female twin cohort.
Third, all of the participants in this study were unselected twins from a population-based registry. In addition to the cannabis use, abuse, and dependence examined in this report, a number of subjects also experienced other substance use problems. For example, of those in our sample who met criteria for cannabis abuse but not dependence, 44% met criteria for abuse of one or more other illicit psychoactive substances, while 36% of individuals who met criteria for cannabis dependence also met criteria for dependence on another illicit substance. We will, in future reports, explore the relationship between the genetic and environmental risk factors for the range of licit and illicit psychoactive substance use disorders.
Finally, the human twin study is a quasi-experimental method that cannot approach laboratory studies in their degree of methodologic rigor (
26). While we have been careful in the design and analysis of this study, we cannot rule out the possibility that our results are influenced by hidden biases.