Identification of impaired cognitive functions is only an initial step in the understanding of the pathophysiology of schizophrenia. The natural second step entails study of the intrinsic neural mechanisms that are associated with these impairments
(6,
7). One approach, used by numerous investigators, seeks to associate individual schizophrenic symptoms and cognitive dysfunctions with specific brain regions
(8–
11). More recently, we and others
(3,
12–
14) have chosen to pursue a strategy that posits the disruption of one fundamental cognitive process that defines the phenomenotype of schizophrenia and affects multiple cognitive domains. If this more parsimonious hypothesis proves to be correct, the heterogeneous array of psychological dysfunctions in schizophrenia may be explained by a disruption in a fundamental cognitive process that can be attributed to a dysfunctional neural circuit
(14).
In light of the fundamental nature of memory in human cognition and earlier reports of marked memory impairment in schizophrenia in relation to performance on other cognitive tests
(5,
15), we have focused our efforts on developing an understanding of the neural mechanisms of human memory. Frontal, thalamic, and cerebellar regions may constitute a circuit that is the core network used by the human brain to perform a variety of memory tasks
(16–
20). Structural and functional abnormalities of the frontal lobe, thalamus, and cerebellum have been demonstrated in schizophrenia
(7–
11,
21–
26). The emerging body of studies showing the role of the cerebellum in several cognitive processes have led to a great interest in understanding its role in psychiatric illnesses
(16,
17,
27,
28).
RESULTS
The patients and control subjects did not differ significantly in task performance either during the practiced condition (mean=12.2 words, SD=4.0, versus mean=14.2 words, SD=2.9) (t=1.40, df=25, p=0.17) or during the novel condition (mean=5.4 words, SD=1.9, and mean=6.6 words, SD=1.5, respectively) (t=1.76, df=25, p=0.09). Therefore, it is unlikely that between-group differences in blood flow were due to differences in task performance.
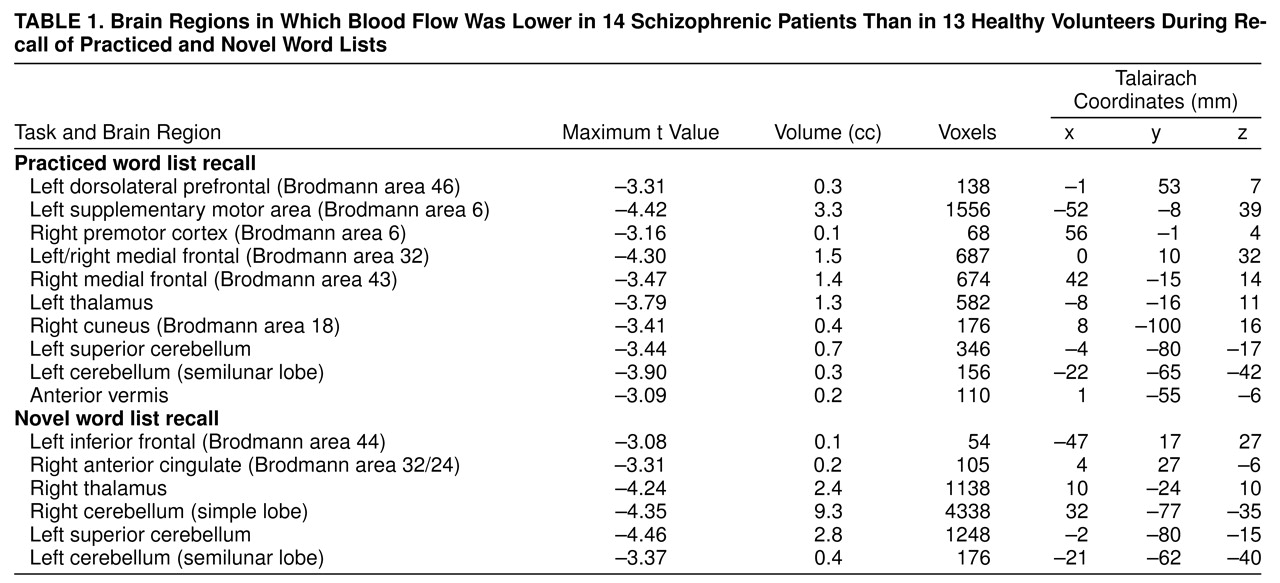
Results of the randomization analysis comparing the patients and control subjects during the practiced task are shown in
table 1 and displayed in
figure 1a. The patients showed a relative decrease in flow in the left dorsolateral prefrontal cortex (Brodmann area 46), bilateral medial frontal cortex (Brodmann areas 32 and 43), left supplementary motor area (Brodmann area 6), right premotor cortex, left thalamus, left cerebellar regions, anterior vermis, and the right cuneus. Similar results were obtained by using the randomization method without subtracting the REST condition. The patients showed a relative decrease in flow in the right frontal cortex, anterior and posterior cingulate, and left cerebellar regions.
Results for the novel memory task are shown in
table 1 and
figure 1b. Brain regions showing a relative decrease in flow in the patients included the left inferior frontal cortex (Brodmann area 44), right anterior cingulate, right thalamus, and bilateral cerebellum (left greater than right). Prefrontal flow decreases were not observed in this analysis when the p<0.005 threshold was used. In an exploratory post hoc analysis we used one-tailed t tests and repeated this analysis using a p<0.01 threshold. At this threshold, a peak of relatively decreased flow was found in the right prefrontal lobe (Brodmann area 47/11) (x=20, y=22, z=–14). Identical results were found when we used between-group comparisons without subtracting the REST condition. The patients showed a relative decrease in flow in the right anterior cingulate, thalamus, and cerebellum.
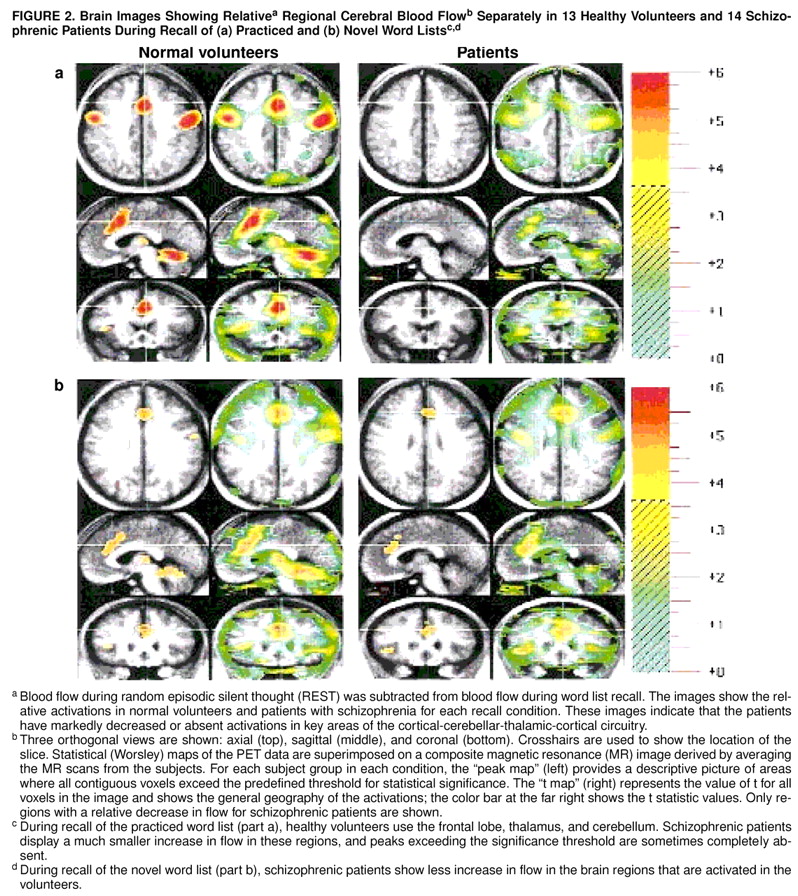
We also examined the results for both cognitive tasks for each subject group independently, using the standard Montreal (Worsley) method for subtracting two experimental conditions
(39). In this analysis we examined practiced recall minus REST and novel recall minus REST in the healthy volunteers and in the patients. Comparison of the activation patterns in these analyses revealed that schizophrenic patients fail to activate brain regions that healthy volunteers utilize during performance of the two memory tasks (
figure 2). Thus, convergent statistical analysis using three different methods confirms that patients with schizophrenia have abnormalities in cortical-cerebellar-thalamic-cortical circuitry.
Only one area with a relative increase in blood flow, the left parietal lobe, was found in the patients with schizophrenia during either memory task.
DISCUSSION
The present study has shown that, compared to healthy individuals, patients with schizophrenia who recall a list of words fail to activate frontal-cerebellar-thalamic circuitry. These findings confirm the hypothesis, based on our previous PET studies, that patients suffering from schizophrenia have a relative decrease in CBF in the same interconnected cerebral regions while performing different types of cognitive tasks
(7,
41). As anticipated, abnormalities in the cortical-cerebellar-thalamic-cortical circuitry are generalized across a variety of memory tasks and are not task-specific.
Lower flow during the practiced task was observed in the left dorsolateral prefrontal cortex (Brodmann area 46), left supplementary motor area (Brodmann area 6), and right premotor cortex. The prefrontal cortex has a pivotal role in carrying out higher cognitive functions by virtue of its reciprocal connections with other brain regions
(42,
43). The results from the present study are consistent with the construct of frontal lobe dysfunction in schizophrenia
(11,
44–
)47). The dorsolateral prefrontal cortex (Brodmann area 46) has been associated with verbal working memory and with the generation of willed actions in healthy individuals
(20,
48). It has also been hypothesized that a defect in working memory function or a disorder of willed actions could be at least one of the crucial cognitive impairments in schizophrenia
(3,
49). Taken together, these results allow us to suggest that a basic impairment of the prefrontal cortex may lead to a core cognitive disturbance in schizophrenia that is shown in several cognitive domains (i.e., working memory or generation of willed actions)
(14).
The lack of differences between patients and control subjects in prefrontal lobe regional CBF during the novel task performance was an unexpected finding. This may be because one strategy used during the novel recall task is the maintenance of the verbal information in an active state (short-term maintenance of verbal information), which has been shown to activate dorsolateral prefrontal cortical areas
(50). This may be why schizophrenic patients display a close-to-normal pattern of prefrontal cortex activation in response to the challenge of the higher prefrontal requirement allocated to task performance. If so, the two groups would not differ in this region when our standard significance threshold is used, although differences might emerge at a lower one. Because our search was hypothesis-driven by our previous work, we examined results obtained by using a lower threshold. With this lower threshold (p<0.01), the schizophrenic patients did show a relative decrease in regional CBF in the right prefrontal cortex.
Another striking finding of our study is the relative decrease in CBF in a large area that encompasses the left lateral and medial rostral supplementary motor area (Brodmann area 6). The rostral supplementary motor area is critically involved in internal representation of time and in internal selection of movement, and it is activated during “complex” tasks requiring selection of response
(51,
52). Although little is known about changes in the supplementary motor area in schizophrenia, relatively decreased activation of the supplementary motor area in schizophrenic patients during motor tasks has been reported
(53).
Decreased thalamic regional CBF in schizophrenic patients was observed on the right for the novel task and on the left for the practiced task. Neuropathological
(23,
54), in vivo anatomical
(24,
55), and functional
(7,
56) studies have shown thalamic abnormalities in schizophrenia. A defect in information processing capacity and in filtering input stimuli has been proposed as one component of the core underlying deficit in schizophrenia
(13,
57). Because of the role of the thalamus in modulating attention and in filtering external information in order to exclude redundant or distracting stimuli, it has been suggested that thalamic dysfunction might play a crucial role in the etiopathological model of schizophrenia
(24,
58).
The patients with schizophrenia also showed a relative decrease in flow in the right anterior cingulate during performance of the recall task involving the novel word list. The anterior cingulate has an important role in emotional and attentional mechanisms
(59), which are also altered in schizophrenia
(4,
60). The healthy individuals showed greater neural activation in the anterior cingulate cortex during performance of the novel task, which has a higher attentional demand than do practiced tasks
(17,
61). Low flow or activity in the anterior cingulate has been reported in schizophrenic patients during several cognitive tasks
(7,
62,
63). Our results showing a decreased flow in the right anterior cingulate, right prefrontal cortex, and right thalamus only during the novel condition suggest that schizophrenic patients may have an inability to activate a right attentional circuit during memory tasks for which the attentional demands are also high.
Multiple cerebellar regions were found to have a relative decrease in flow in schizophrenic patients during both conditions. Our group
(7,
41) has previously demonstrated that schizophrenic patients display relatively lower blood flow in diverse cerebellar regions during the recall of complex narratives and during recognition memory for words. Intriguingly, neuroleptic-naive schizophrenic patients had a relative increase of CBF in the cerebellum when they were examined in a REST condition
(46).
It has been proposed that a cerebellar dysfunction in schizophrenia may lead to “poor mental coordination,” resembling abnormalities in motor coordination and sequencing
(64). Results from the present study also show that patients with schizophrenia have a relatively lower blood flow during recall of a practiced word list in the anterior vermis. Interestingly, some anatomical studies
(22,
65) have shown a smaller anterior vermis in schizophrenia.
Historically, dysmetria has been defined as the inability of the individual to time the control at onset and offset of activity in the appropriate pairs of muscles. Dysfunction in the central timing process has been attributed to the lateral cerebellum, the putamen, and the supplementary motor area
(64,
66). It seems possible that these brain regions related to the timing and programming of movement may also be involved in timing and controlling the fluid coordination of mental activity
(67–
69).
A dysfunctional circuitry linking brain regions involved in timing and sequencing mental functions (i.e., cerebellum and rostral supplementary motor area) to regions involved in high-level cognitive processes (i.e., prefrontal lobe) provide strong support for a “cognitive dysmetria” model of schizophrenia. This model posits that a neural misconnection in cortical-cerebellar-thalamic-cortical circuitry in schizophrenic patients may lead to difficulty in coordinating and sequencing mental processes, such as receiving, processing, retrieving, and expressing information, that are needed to achieve desired cognitive acts. While this circuit is hypothesized to be impaired across a broad range of cognitive tasks, specific differences in regional CBF patterns across different types of cognitive tasks are also expected, since different interconnected brain regions could be recruited according to specific features of the cognitive task. On the basis of these findings it is possible to speculate that the clinical heterogeneity of schizophrenia can be understood in the context of dynamic shifts in this disturbed circuitry, whereas the disturbed circuitry represents a basic neural phenomenon common to patients suffering from schizophrenia.