Recent investigations have postulated a relationship between signal hyperintensities on T
2-weighted magnetic resonance scans and geriatric depression
(1–
5), although hyperintensities have also been reported in normal elderly subjects
(5–
8). The prevalence of these signal hyperintensities increases with age
(5,
9–
11), and they are more prominent in subjects with cerebrovascular disease risk factors
(8,
12–
14) and, in some reports
(5,
15), in subjects with dementia.
An extensive neuropsychological body of evidence in depression and geriatric depression demonstrates cognitive deficits that are reversible following successful somatic treatment of the depression
(26–
30). This phenomenon, variably called “dementia syndrome of depression,” “depressive pseudodementia,” and “cognitive impairment of depression”
(31–
33), may preferentially occur in elderly as compared to younger depressed subjects
(33), although dissenting findings exist
(26). The neuropsychological characteristics of late-life cognitive impairment of depression have been described as “subcortical”
(34), in that the cognitive deficits observed resemble the deficits seen in neuropsychiatric conditions characterized by subcortical, rather than cortical, pathology. Furthermore, cognitive deficits in depression have been linked to subcortical-frontal lobe abnormalities
(23,
35). Taken together, such data have contributed to a “subcortical dysfunction model” of learning and memory in affective illness
(36–
38).
The purpose of this study was to extend examination of the relationship among signal hyperintensities, depression, and cognitive performance. Hyperintensity ratings in different brain locations and neuropsychological measures addressing different cognitive domains were evaluated in depressed and normal comparison subjects in whom dementia or a history of transient ischemic attack or stroke had been ruled out. We hypothesized that poorer cognitive performance would be associated with more severe hyperintensities.
METHOD
Subjects
Forty-one elderly depressed patients participated in the study. The patients were recruited from the Geriatric Psychiatry Service (inpatient, outpatient, and day hospital) at Hillside Hospital. Thirty-eight normal comparison subjects recruited from the community were solicited by means of advertisement in local newspapers or by word of mouth. All subjects received a Structured Clinical Interview for DSM-III-R (SCID)
(41), were age 65 years or older, and were right-handed. Patients met the following inclusion criteria: DSM-III-R criteria for major depression, unipolar, as determined by the SCID and a score of 18 or greater on the 21-item Hamilton Depression Rating Scale
(42).
Exclusion criteria for patients and comparison subjects included presence of a cardiac pacemaker, metallic clips, or other bodily metallic implants or artifacts (because of the magnetic resonance imaging [MRI] procedure); presence of significant acute medical illness or exacerbation of a chronic medical condition; presence of a neurodegenerative disorder, including Alzheimer’s disease or a related dementia (subjects did not meet DSM-III-R criteria for dementia); history of transient ischemic attack or stroke; and other past or current DSM-III-R diagnoses (for comparison subjects this included affective disorders). After complete description of the study to subjects, written informed consent was obtained.
The Clinical Global Impression for depression
(43) and the Hamilton Depression Rating Scale
(42) were administered to all subjects. Both psychiatric ratings were completed by an experienced geriatric psychologist (E.K.-G.) with demonstrated high interrater reliability
(44). Other clinical and demographic data were assessed through use of a standardized format for information collection.
MRI Image Acquisition
Subjects were scanned in a 1.0-T whole-body MRI system (Siemens Magnetom). T2-weighted and proton density (intermediate) brain images were obtained in the axial plane. This series had a repetition time (TR) of 2500 msec and an echo delay time (TE) of 25 and 90 msec. The sequence produced 20 parallel sections in a 256×256 matrix with a 1.3 zoom factor. Axial images were 7 mm thick with a 0.7-mm gap between each section. A full coronal series was also obtained but was not used for the ratings.
MRI Scan Analysis
Hard copy images were printed for visual quantitative evaluation of signal hyperintensities. MRI scans of patients and comparison subjects were combined in a randomized order and evaluated under blind conditions by a research psychiatrist (K.R.R.K. or B.S.G.). Hyperintensities were assessed according to the modified Fazekas criteria
(45). The modified Fazekas gradings follow an ascending degree of severity and frequency of hyperintensities (gradings=0–3) and rate them in three brain locations: periventricular region, deep white matter, and subcortical gray matter.
Interrater reliability for hyperintensity ratings were established by using intraclass correlation coefficients (periventricular hyperintensities ICC=0.86, deep white matter hyperintensities ICC=0.87, and subcortical gray matter lesions ICC=1.00).
Neuropsychological Measures
A neuropsychological test battery was administered to assess and quantify cognitive performance. On the basis of previous studies
(24,
46,
47), selected tests were chosen from several domains for analyses. The battery consisted of tests representative of subcortical and cortical cognitive domains and included visuospatial functioning (block design of the WAIS-R
[48]), attention/information processing (digit symbol of the WAIS-R
[48], attention subscale of the Dementia Rating Scale
[49]), language (Boston Naming Test
[50]), executive functioning (initiation/perseveration subscale of the Dementia Rating Scale
[49], Animal Naming Test
[51], Controlled Oral Words Association Test
[52]), and memory (Wechsler Memory Scale—Revised
[53]). Both general memory and delayed recall memory index scores were derived according to the method of Woodard and Axelrod
(54). The Dementia Rating Scale
(49) was used as an overall measure of cognitive functioning. Because of occasional uncooperativeness, not all subjects completed every neuropsychological test.
Statistical Analysis
Clinical and demographic differences between depressed patients and comparison subjects were analyzed by using independent t tests and chi-square tests. To clarify the relationship among the regional distribution and severity of hyperintensities, neuropsychological function, and diagnosis of depression, the depressed and comparison subjects were dichotomized for the different brain region (periventricular, deep white matter, and subcortical gray matter) hyperintensity ratings into none/minimal (0–1 rating) and moderate/severe (2–3 rating). In this way each neuropsychological factor was then analyzed separately, with a main effect for group (depressed, comparison), a main effect for hyperintensity rating severity (in each brain area), and an interaction effect (group-by-hyperintensity ratings per area) being derived. Analysis of covariance (ANCOVA), with adjustment for the effects of age and years of education, was used to study both the main effects of group and hyperintensities, as well as their interactions, on relevant neuropsychological summary scores. Separate ANCOVAs were performed for periventricular, deep white matter, and subcortical gray matter regions. When significant interactions were observed, post hoc analyses of the cell means were performed. Because of the high number of ANCOVAs and increased chance of type II error, results were considered significant only if they met alpha criterion of 0.01. Two-tailed tests of probability were used to estimate statistical significance in all analyses.
RESULTS
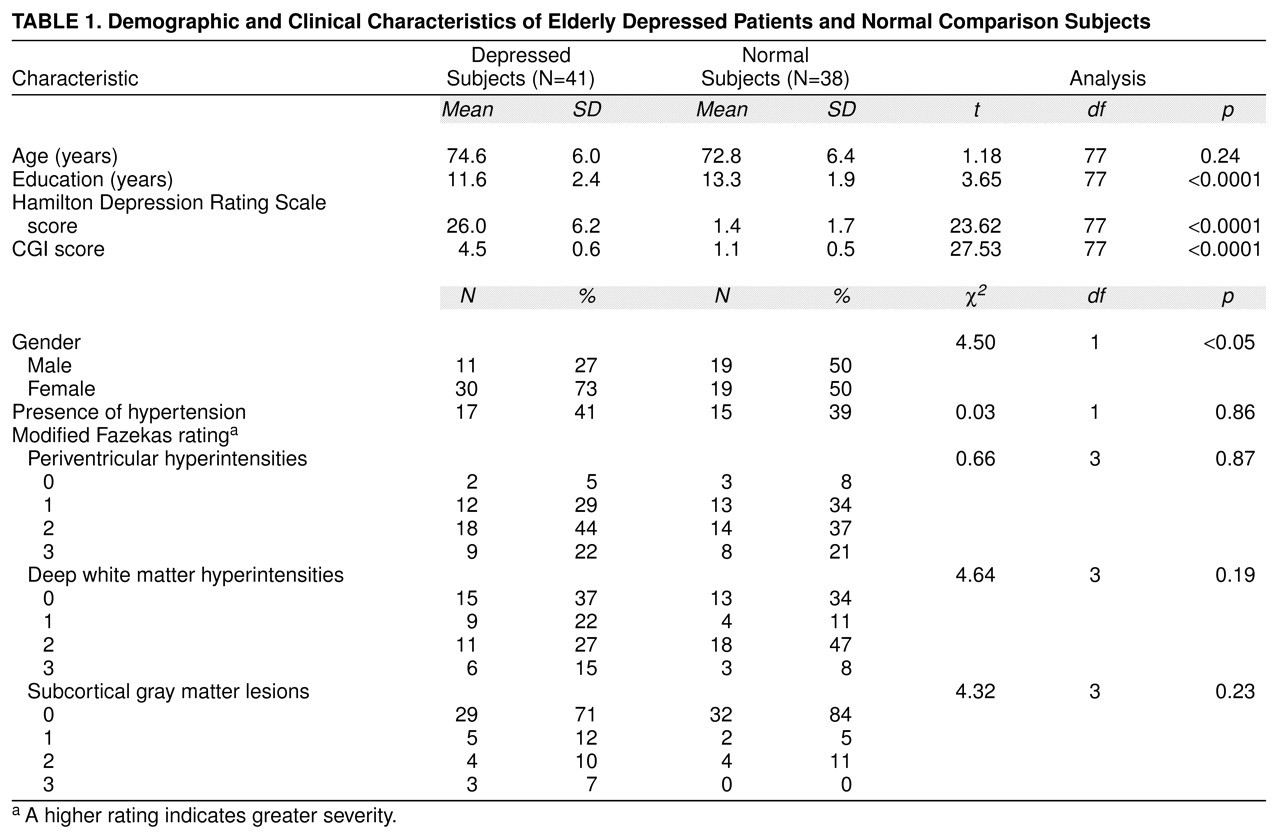
Age, sex distribution, years of education, depression ratings, and distribution of hyperintensity severity ratings are compared for depressed and normal subjects in
table 1. Depressed patients were slightly, but nonsignificantly, older than normal comparison subjects. Although a higher proportion of men made up the comparison group, t test analyses revealed no significant differences between men and women on any of the neuropsychological test scores. Comparison subjects were also somewhat more educated than depressed patients. Scores on depression rating scales were, as expected, much higher for patients.
ANCOVA, which controlled for age and years of education, revealed a significant main effect of group on neuropsychological performance. Depressed patients performed worse on several cognitive tests, including general memory index (F=10.08, df=1, 73, p=0.002), block design (F=6.95, df=1, 61, p=0.01), and digit symbol (F=8.14, df=1, 50, p=0.006) and on the overall cognitive measure (the Dementia Rating Scale) (F=15.32, df=1, 72, p<0.0001). Hyperintensity severity as a main effect was not significant for any cognitive measures.
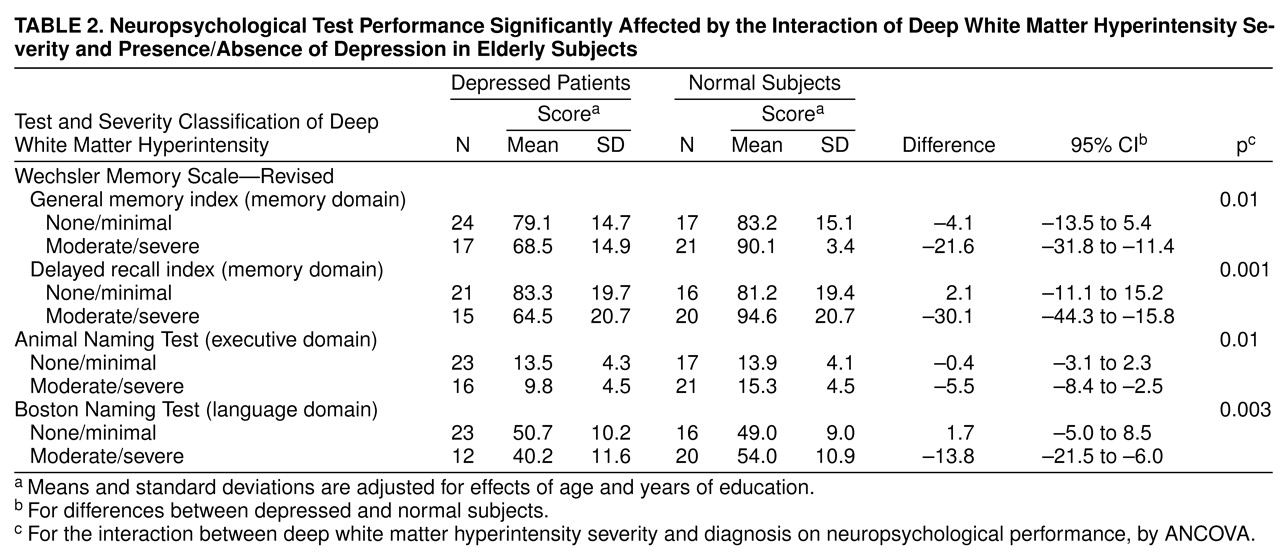
The interaction of group by hyperintensity location was examined to explore the influence of both factors on cognitive performance. Statistically significant differences were obtained as a function of group membership (depressed and comparison groups) and location of hyperintensities. The principal findings were that depressed subjects with moderate-to-severe hyperintensities in the deep white matter (N=17) performed significantly worse than depressed patients without such lesions (N=24) or comparison subjects with (N=21) and without (N=17) moderate-to-severe deep white matter hyperintensities on the following cognitive measures: general memory index (F=6.73, df=1, 73, p=0.01), delayed recall index (F=11.77, df=1, 66, p=0.001), Boston Naming Test (F=9.44, df=1, 65, p=0.003), and Animal Naming Test (F=6.84, df=1, 72, p=0.01).
Table 2 presents age- and education-adjusted means for these tests. Ninety-five percent confidence intervals (CIs) for mean neuropsychological test differences between depressed and comparison subjects in both hyperintensity groups (i.e., none/minimal and moderate/severe) are presented. In contrast, no significant interaction effects were observed for group by hyperintensities in periventricular or subcortical gray matter areas.
DISCUSSION
The results of this study validate other reports that greater cognitive deficits exist in elderly depressed patients than in age-similar normal subjects and additionally suggest that deep white matter pathology may relate to some of these deficits. Poorer test performance in depressed patients than in comparison subjects occurred in several neuropsychological domains including attention/information, visuospatial, and memory processing, as well as overall cognitive functioning. The literature addressing cognitive dysfunction in depression ranges from studies reporting an absence of any cognitive changes
(55–
57) to those reporting severe changes
(36,
38,
58, >
59). The results in the present study concur with studies reporting abnormalities in visuospatial functioning
(24) and verbal
(27,
60,
61) and visual
(27,
62,
63) memory impairment in depressed as compared to normal subjects. Findings in the literature regarding attentional performance
(64) and language processing
(65–
68) are, however, more controversial. Not all studies of cognitive impairment in depression have examined a clear geriatric-only group. The findings in our study indicate that cognitive impairment in geriatric-only depressed patients cuts a fairly wide swath across multiple neuropsychological domains.
Evidence in prior reports is conflicting regarding the significance of MR signal hyperintensities on cognition in depressed and nondepressed geriatric populations. In normal elderly subjects, current findings reconcile with many studies that have failed to demonstrate a relationship between hyperintensities and cognitive performance
(19–
22). On the other hand, other investigations have demonstrated a relationship between hyperintensities and impaired attention, visuospatial functioning, and speed of processing
(16–
18). Some investigations of depressed patients have reported that patients with deep white matter lesions did not differ in terms of cognitive performance from those without such lesions
(5,
47), whereas others have found an association between impairments in mental speed, executive functioning, and memory and presence of deep white matter
(23–
25), periventricular
(20), and subcortical gray matter
(69) hyperintensities. This lack of consensus among studies may reflect differences in subject groups, cognitive tests administered, and MRI procedures/ratings; but it also likely is a consequence of the tremendous clinical heterogeneity of older depressed groups
(70).
The present study differs from most other studies of elderly unipolar depressed subjects in that hyperintensities were rated in different brain regions (periventricular, deep white matter, subcortical gray matter) for the same patients, allowing simultaneous evaluation of the interaction of cognitive performance and distribution/severity of hyperintensities. In this study, less severe hyperintensities in all three brain regions in depressed and comparison subjects, and more severe hyperintensities in periventricular and subcortical gray regions in both groups, did not significantly influence cognitive performance. However, findings suggest that there is an interaction between the presence of depression and more severe signal hyperintensities in deep white matter that is associated with or hypothetically may mediate several, but not all, of the cognitive deficits observed in the depressed group. Of interest is that particular neuropsychological areas that were implicated (e.g., executive functioning, memory) are functions traditionally ascribed to subcortical/frontal brain systems
(16,
36,
37) that have themselves been associated with an emerging neuroanatomy of depression
(3,
39,
71–
73). We hypothesized that hyperintensities would be associated with cognitive impairment; however, only deep white matter changes were relevant in this study. Neuroanatomically, this suggests that interruptions between key cortical and subcortical gray matter regions/structures in the mood regulatory circuitry (i.e., in white matter tracts) may be more pertinent to cognitive deficits in geriatric depression than small, discrete lesions in the gray matter structures themselves (e.g., basal ganglia, thalamus), even though hyperintensities in both central gray and deep white matter regions have been linked to depression either as a neurobiological correlate or as a susceptibility factor
(1–
5,
15,
23,
69,
74,
75).
Furthermore, because more severe hyperintensities in deep white matter probably represent cerebrovascular disease changes histopathologically
(76,
77), current data implicating moderate to severe—but not minimal—hyperintensities in the executive and memory function deficits seen in depressed subjects suggest that a threshold level of ischemic pathology in deep white matter may be necessary for these neuropsychological impairments to emerge during depression. Because similar deep white matter hyperintensity changes in elderly normal subjects were not significantly associated with any cognitive deficits, it appears that depression represents a vulnerability to or necessary cofactor for cognitive compromise associated with deep white matter hyperintensities.
Hyperintensities occur in deep white matter that contains tracts connecting subcortical and cortical brain structures such as the basal ganglia and frontal lobes in which depression state-dependent hypometabolism has been demonstrated in functional neuroimaging studies
(78,
79). Furthermore, ascending neurotransmitter systems implicated in depression modulate these structures/circuits as well
(3,
80,
81). As such, although deep white matter pathology itself may predispose an individual to depression, cognitive expression of deep white matter abnormalities may be contingent on a concurrent or “superimposed” state-dependent hypometabolism in similar or connected brain regions and/or a neurochemical disequilibrium associated with depression. In this way, more severe deep white matter changes may contribute to the so-called reversible cognitive impairment of depression (or dementia syndrome of depression or depressive pseudodementia)
(31–
33). This notion thematically resonates with the finding of regional cerebral blood flow (CBF) abnormalities in depression with “additive” further regional CBF abnormalities in those depressed subjects with reversible cognitive impairment
(82). Future studies that combine similar neuroimaging assessments with neuropsychological testing during depression and after recovery will help determine whether deep white matter changes in elderly depressed subjects are also associated with postrecovery evidence of persisting, but lesser, cognitive deficiencies—a phenomenon that has been demonstrated in a substantial proportion of remitted elderly depressed subjects who had experienced more evident cognitive difficulties when depressed
(83).
Current findings suggest a possible brain substrate for some of the often reversible cognitive deficits encountered in elderly depressed patients. However, the MR hyperintensity rating methodology employed in this study scored hyperintensities regardless of where they occurred in the subcortical deep white matter (i.e., frontal, parietal, occipital, temporal regions). As such, further speculation on brain-behavior relationships are dependent on rating methods that more specifically localize hyperintensities in the brain. We are presently analyzing data based on more neuroanatomically specific ratings
(74) in a different population of older depressed subjects and comparison subjects who also underwent an identical neuropsychological battery.