Patients with schizophrenia, as a group, experience a wide range of neurocognitive deficits. Some of these deficits may be linked to the symptoms of the disorder, whereas others occur even when symptoms are absent and appear to be linked to vulnerability to schizophrenia
(1,
2). Deficits in early visual perception that are assessed with visual masking techniques may indicate vulnerability to schizophrenia. In visual masking, the visibility of a briefly presented target is reduced by a mask that is presented very shortly before or after the target
(3,
4). Generally, subjects can detect the target accurately when it is presented by itself. However, the additional presence of the mask makes it harder to identify the target. When the target and mask overlap the retinal location, the effect is called backward masking. Patients with schizophrenia consistently show backward masking performance deficits in that they require a longer interstimulus interval between target and mask to identify the target than do nonclinical subjects
(5–
9).
Performance deficits on backward masking tasks cannot be explained by antipsychotic medications, which instead appear to help reduce the deficit
(10) or have no effect
(11). Masking performance seems rather independent of psychotic symptoms because it does not correlate with the ratings of psychotic symptoms such as hallucinations and delusions. In contrast to this independence from positive symptoms, masking performance appears to be related to formal thought disorder, as measured by the Ego Impairment Index
(12,
13), and to negative symptoms
(6,
14–
16). Poor premorbid functioning
(17), poor prognosis
(8), and greater chronicity
(7) have also been linked to masking performance deficits. Performance deficits are often, but not always, found among nonpsychotic individuals who are considered to be psychosis prone because of clinically assessed symptoms or scores on psychometric tests
(18–
21). Findings on these psychosis-prone individuals indirectly suggest that deficits involving masking performance may be indicators of vulnerability to schizophrenia.
Two other types of designs provide more direct evidence as to whether performance deficits reflect a vulnerability to schizophrenia as opposed to reflecting symptoms of the illness: 1) a test of first-degree relatives of patients with schizophrenia and 2) a test of patients with schizophrenia in clinical remission. In a previous study, we reported that unaffected siblings showed deficits across several backward masking conditions and the differences were particularly pronounced in the early (sensory-perceptual) part of the masking function
(22). In the current study, we extended this line of investigation by testing unmedicated patients with schizophrenia during a remission of psychosis. Backward masking performance deficits in such remitted patients would constitute converging support for the hypothesis that masking deficits indicate vulnerability to schizophrenia. A previous study of backward masking in remitted patients with schizophrenia
(23) reported masking performance deficits in 10 remitted patients compared with 10 matched normal subjects. While it is highly suggestive, that study had two potential limitations. One was that the degree of remission was not documented by using any symptom rating scale. Thus, it is entirely possible that the patients were in partial, as opposed to full, psychotic remission. A second possible limitation was that the remitted patients were medicated, although, as mentioned, it is unlikely that medications caused this deficit. We tried to address both of these issues by recruiting patients who were in carefully documented psychotic remission and who were in a period of no medication use.
A separate goal was to determine whether the deficits in backward masking are consistent with underlying deficits in cortical oscillation. It is possible that a variety of perceptual deficits can be explained in a more parsimonious manner by abnormalities in cortical oscillation. Oscillations in the gamma range (30–70 Hz) are found over multiple cortical areas of several species
(24) and are thought to be involved with feature binding and visual attention
(25–
27). In a recent study, Clementz et al.
(28) proposed that deficits in patients with schizophrenia on a measure of sensory gating (P50) may be associated with suppression in cortical oscillations in the gamma range. Because the P50 procedure is conceptually related to visual masking
(5), it raises the question of whether deficits in masking are also linked to cortical oscillations.
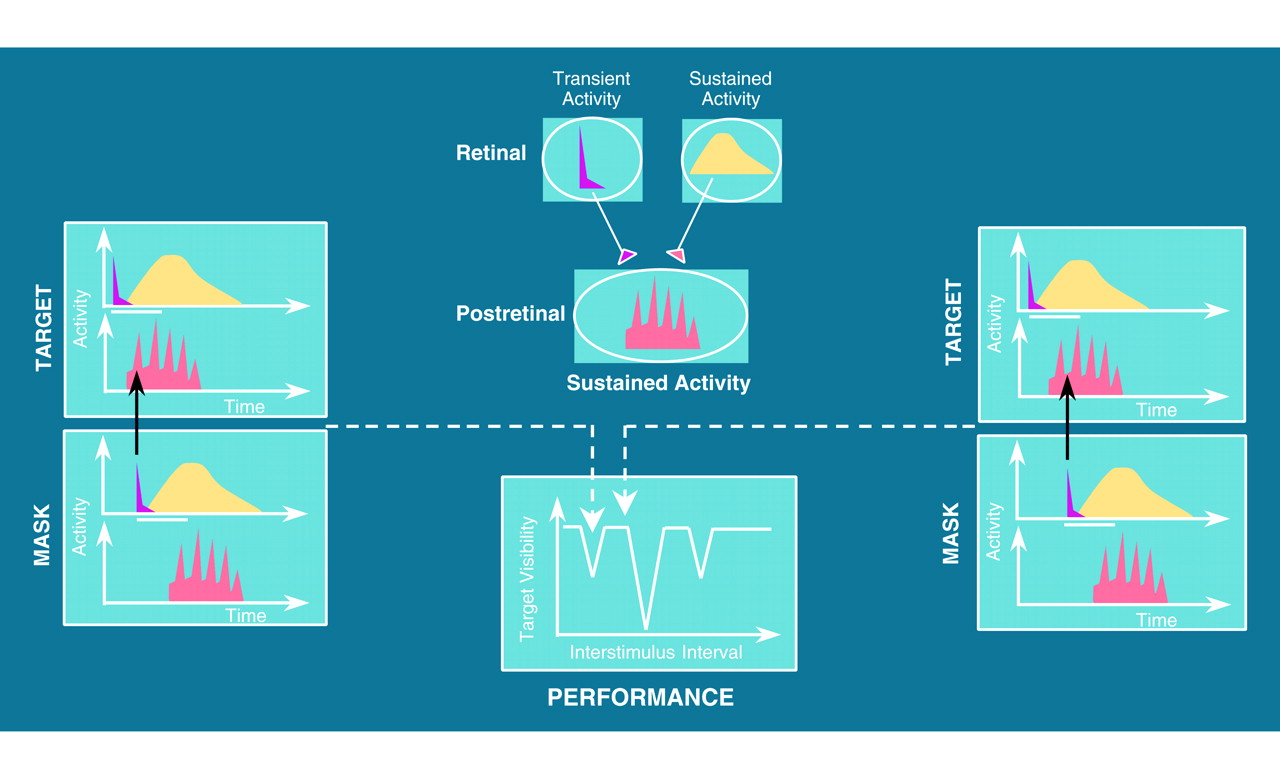
Backward masking lends itself well to investigations of neural processes and pathways. Our initial interest in applying visual masking procedures to schizophrenia stemmed from a model by Breitmeyer and Ganz
(4), which proposed that visual masking depended on the interactions of two types of visual pathways: transient and sustained. These two pathways form the basis of the complex visual tasks conducted in the cortex, such as object recognition and spatial relationships among objects
(29). The two pathways run in parallel from the retina, with little interaction until they reach area V1 in the cortex, which is the proposed earliest level for the masking effect
(3).
Components of this model have received direct experimental support. First, data from intracranial recordings of the visual system of the cat revealed that sustained channels indeed show oscillating activity in the gamma range and, further, that brief transient activity can inhibit the activity of the sustained channels
(31). Second, a masking study with humans that used frequent measurements at brief interstimulus intervals observed an oscillating performance pattern
(30). Hence, an additional goal of this study was to incorporate these recent developments in masking models to help interpret schizophrenic masking deficits. That is, are patterns of masking performance in schizophrenia consistent with abnormalities in cortical oscillations?
METHOD
Subjects
The subjects were 11 patients with recent-onset schizophrenia from the University of California at Los Angeles Aftercare Program who, at the time of assessment with the masking procedures, were in a clinically stable state and who had not been administered depot or oral antipsychotic medications for at least 3 months. Patients were eligible for entry into the Developmental Processes in Schizophrenic Disorders project at the Aftercare Program if they were within 2 years of the onset of their first psychotic episode. Subjects in this group received a diagnosis of schizophrenia or schizoaffective disorder, mainly schizophrenic, according to the Research Diagnostic Criteria
(32). Full-group selection criteria for that project were presented in a report by Nuechterlein et al.
(33). All patients in this study were in psychotic remission, defined as having no score in the psychotic range on hallucinations, unusual thought content, or conceptual disorganization items from the expanded version of the Brief Psychiatric Rating Scale
(34). In addition, 10 of the patients had no negative symptoms in the clinical range, defined as having no score above 3 on any negative symptom item (blunted affect, motor retardation, and emotional withdrawal). These patients with recent-onset schizophrenia were participating in a trial period without medication, after at least a year of maintenance antipsychotic medication, to determine whether ongoing maintenance was needed (except for one subject who self-initiated a medication discontinuation)
(33).
The 11 matched comparison subjects were selected from a larger group of subjects who had participated in a previous study and had experienced the same test procedures. Comparison subjects were excluded for history of any psychotic disorder, bipolar disorder, schizotypal or paranoid personality disorder, substance dependence, recurrent depression, or presence of a first-degree relative with schizophrenia (see reference
22 for full selection criteria). The comparison subjects were matched to the patients for gender, age, and education. Each group contained nine men and two women. Mean ages were 24.4 and 24.9 years, and mean education was 14.0 and 13.4 years for patients and comparison subjects, respectively. Written informed consent was obtained from all subjects after the procedures were explained.
Procedure
Three conditions were administered that were used in previous studies of masking with patients with chronic schizophrenia and their siblings
(35,
36). These conditions were initially selected because of their differential reliance on sustained and transient visual channels. Subjects sat 1 m away from a rear-projection screen and were tested on a three-channel Gerbrands (Arlington, Mass.) projection tachistoscope (model G1176). A fixation point was presented for 400 msec and ended 200 msec before the target presentation. Next, one of four letters (S, C, O, or Q) was presented at any one of four locations that were 2.2˚ of visual angle away from the fixation point (top, right, bottom, or left). Target letters subtended 0.3˚ of visual angle. The mask consisted of four clusters of overlapping Xs, which covered each of the four possible target locations. During administration of the examination, an experimenter sat on the other side of the rear-projection screen and could see whether the subject was looking at the screen. Luminance was determined with a light meter held 1 m from the screen at eye level. Subjects were tested in a lighted room with ambient light at approximately 111 cd/m
2 (with a light diffuser). Target and mask displays (stimuli and background) were both presented at a luminance of approximately 732 cd/m
2 (without a light diffuser), and the luminance of the stimuli alone was approximately 9.5 cd/m
2. Presentation order of the target letters and of their locations was randomly assigned.
During practice, the target was first presented without the mask for 10 msec. All subjects were able to identify the target presented alone on at least eight out of 10 trials. Next, subjects practiced with both target and mask at an interstimulus interval of 100 msec, the longest interval within our masking functions and an interval for which we did not expect much masking on the basis of previous studies. Subjects were required to correctly identify at least four out of five targets at this interstimulus interval before proceeding with the test.
Three masking conditions were administered in a counterbalanced order across subjects. Interstimulus intervals were set at 5, 10, 20, 40, 70, and 100 msec, with 12 targets presented at each interstimulus interval. Both the targets and the interstimulus intervals were presented in a block-randomized fashion, with the constraint that the same interstimulus interval could not be presented twice in succession. The conditions included the following.
1. Target identification with a high-energy mask. In this condition, subjects identified a clearly focused target with high spatial frequency information. The energy of the mask was greater than the energy of the target. The term “energy” in this context refers to the light intensity times the duration of the stimulus. A high-energy mask typically generates a monotonic masking function in which performance gradually improves as the interstimulus intervals become longer. In this condition, the energy of the mask was twice the energy of the target (20 msec for a 10-msec target).
2. Target identification with a low-energy mask. In this condition, subjects also identified a clearly focused target. It was designed to generate a nonmonotonic masking function in which performance does not regularly improve as the interstimulus intervals become longer (e.g., U-shaped). In this condition, the energy of the mask was one-half the energy of the target (5 msec for a 10-msec target).
3. Target identification of a blurred target. In this condition, subjects identified a target that was blurred to a standardized degree by changing the lens-to-screen distance from 95 cm to 107.5 cm (which defocused the stimuli) and then refocusing the mask but not the target. As a result of blurring, visual discrimination became more difficult. To obtain a performance comparable to that on the low-energy condition and to prevent floor effects, the duration of the target was doubled (from 10 to 20 msec) and the duration of the mask was set to one-fourth of the duration of the target (5 msec for a 20-msec target).
Statistical Analysis
Data were analyzed by using a two-(diagnostic group)-by-three-(condition)-by-six-(interstimulus interval) repeated measures analysis of variance. Follow-up analyses of diagnostic differences in the shape of the performance curves used orthogonal polynomial contrasts in the repeated measures. A general increase across interstimulus intervals (reflected in the linear component) was expected in both diagnostic groups and was not a focus of study. Interest centered on the second-degree (U-shaped) and fourth-degree (W-shaped or oscillating) polynomial components. Partitioning the overall diagnosis-by-interstimulus-interval interaction into separate polynomial contrasts provided an opportunity for statistical tests for group differences in these two shape components. Separate within-group analyses, using polynomial contrasts on the repeated measures, were also done within each diagnostic group to further clarify the specific nature of the performance curves. The 100-msec interstimulus interval was omitted from the analyses of the shapes of the curves because performance in both diagnostic groups in virtually all conditions was at maximum. For all tests, the significance level was set at p=0.05, two-tailed.
RESULTS
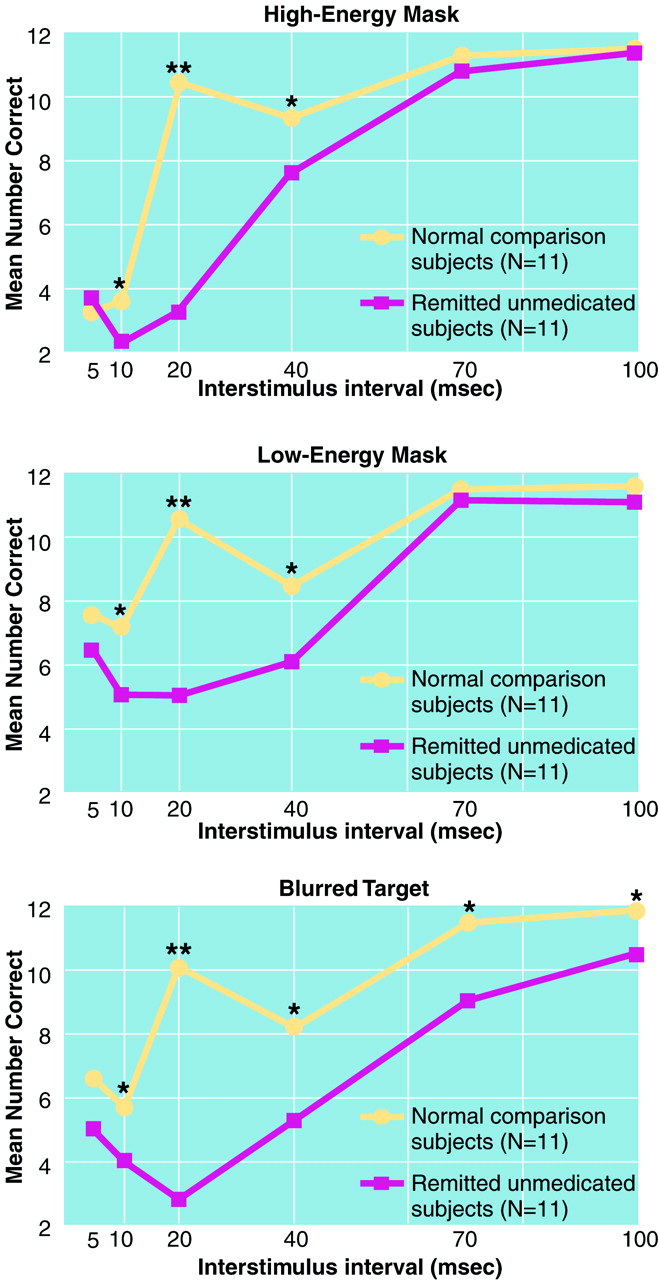
Results are displayed for each of the three masking conditions in
figure 2. Significant group contrasts at each interstimulus interval are shown. The overall effects of group (F=74.17, df=1, 20, p<0.0001) and test condition (F=5.06, df=2, 40, p<0.02) were significant, the group-by-interstimulus-interval interaction was significant (F=27.64, df=5, 100, p<0.0001), but the group-by-condition interaction was not. Hence, the remitted unmedicated patients showed performance deficits across the masking conditions as compared with the matched normal group, and the pattern of performance was different for the groups.
Inspection of
Figure 2 strongly suggests that the two groups differed in the shapes of their masking functions, in addition to overall differences in performance level. To examine the nature of the group differences in the shape of the masking performance function, we conducted between-group contrasts for the second-degree (U-shaped) and fourth-degree (W-shaped or oscillating) polynomial components. The groups differed significantly for the second-degree polynomial component for each condition (all p<0.0002). Also, the groups differed for the fourth-degree polynomial component for each condition (all p<0.02). Next, we conducted within-group analyses to clarify the nature of the between-group differences in the shape of the masking functions. For each masking condition, the patients had a significant second-degree component (all p<0.0002). The second-degree component for the comparison subjects was weaker and nonsignificant for two of the conditions. Each masking function for the matched comparison group included a significant fourth-degree component (all p<0.0005). However, the fourth-degree component was not significant for the patients in any masking condition. Hence, both between- and within-group analyses supported the interpretation that masking functions of the groups differed; the patients showed more U-shaped masking functions, and the comparison subjects demonstrated more oscillating functions.
As a final check to determine whether differences in the shape of the masking function could be explained by a few outliers, we examined the data for each subject individually, in a categorical fashion. A pattern was defined as oscillating if the score at the 20-msec interval was higher than the scores at both the before and after intervals. More comparison subjects than patients showed an oscillating pattern. This difference was highly significant for the high-energy mask (eight comparison subjects versus zero patients; χ
2=12.57, df=1, p<0.001) and for the blurred target (eight comparison subjects versus one patient; χ
2=9.21, df=1, p<0.002). For the low-energy mask, the difference was significant for the chi-square test (seven comparison subjects versus two patients; χ
2=4.70, df=1, p<0.03) but not for Fisher’s exact test (p<0.08), which is known to be more conservative
(37).
DISCUSSION
Patients with schizophrenia in psychotic remission and without antipsychotic medication showed a backward masking performance deficit across several masking conditions compared with demographically matched comparison subjects. The current study supports and extends a previous study with medicated patients in which the degree of remission was not directly measured
(23). In combination with a previous study of unaffected siblings of patients with schizophrenia
(22), these data provide converging evidence that impaired performance on backward masking conditions indexes a vulnerability factor for schizophrenia. The groups in the current study were relatively small, a result of our stringent selection criteria, which required both full psychotic remission and a lengthy interval (at least 3 months) of no medication use.
In our previous study of siblings, we made a rough division between the early, sensory-perceptual and the later, attentional-disengage components of the masking function. The performance deficits of the siblings of patients were especially pronounced in the early, sensory-perceptual part of the masking function. Data from the current study were generally consistent with those of the previous study, because group differences were largest at an early interstimulus interval. When we compared the effect sizes between groups (Cohen’s h
[38]) at each interstimulus interval, the effect sizes at the 20-msec interstimulus interval for each condition were several times larger than those at intervals placed only 5 msec before or 10 msec after the 20-msec interval. Thus, the current data suggest that a distinction between simple, early, sensory-perceptual and later, attentional-disengage components may not fully capture the group differences.
In the context of a general performance deficit, it is still possible for patients with schizophrenia to perform particularly poorly on one test more than other tests for psychometric reasons, as opposed to their having a true differential deficit
(39). Similarly, patients with schizophrenia might perform much worse than the comparison group at the 20-msec interstimulus interval because the psychometric properties at that interval are especially favorable for discriminating patients from comparison subjects. However, we would expect the 40-msec interstimulus interval (about 65%–75% correct) to be a better interval for distinguishing between groups than the 20-msec interstimulus interval (about 85% correct for the comparison group) on the basis of its moderate difficulty level, rather than the reverse. The observed pattern strongly suggests that the test reveals a true difference between the groups at the 20-msec interval.
The fact that the groups differed mainly at the 20-msec interval is attributable to substantial qualitative differences between groups in the shapes of the masking functions: the comparison subjects showed an oscillating performance pattern, but the patients did not. Qualitative differences in the shape of the masking functions were demonstrated both with trend analyses and with categorical analyses. Analyses revealed group differences in both the second- and fourth-degree polynomial components of the masking functions. The greater second-degree component (U-shaped) of the patients in each masking component is consistent with the theory, suggested by us and others, that patients with schizophrenia have overactive transient channels
(35,
40,
41). However, overactive transient channels alone cannot explain why the patients showed less of a fourth-degree component in each condition. To explain this finding, we need to consider oscillating systems.
Backward visual masking can occur when the transient pathways of the mask inhibit the oscillating sustained pathways of the target
(4,
42). As illustrated in
Figure 1, oscillating sustained channels are more or less susceptible to inhibition by the mask, depending on whether the sustained channel activity is at a peak or a trough of its cycle
(30). This cycling in susceptibility should be revealed as a pattern of oscillating masking performance. Because the comparison group, but not the patients, showed an oscillating pattern, our findings are consistent with a basic defect in establishing cortical oscillations in unmedicated patients with schizophrenia who are in psychotic remission.
If masking deficits reflect a failure to establish cortical oscillations, this should be reflected in the masking performance of chronic, as well as remitted, patients with schizophrenia. To address this question, we reanalyzed data from our previous studies that included a larger group of patients with chronic schizophrenia and a larger comparison group
(35,
36). Specifically, we considered whether patients and comparison subjects differed in the fourth-degree polynomial component. Indeed, the patients with chronic schizophrenia showed significantly less of a fourth-degree component in all three of the masking conditions used in the current study (all p<0.0002).
Our confidence in the findings of qualitative differences in performance between patients and comparison subjects is further bolstered by a recent publication from Cadenhead et al.
(43). Using stimuli and task parameters very similar to our own in the low-energy mask condition, they uncovered a highly comparable pattern in which the normal comparison group’s identification performance spiked up at the 20-msec interstimulus interval and then dropped at the 40-msec interval, whereas the performance of patients with schizophrenia did not show such a pattern (demonstrated in figure 3 of Cadenhead et al.).
Normal cortical oscillations in the gamma range (30–70 Hz) may be intrinsic to the cortex, or they may be driven by oscillating input from subcortical structures such as the thalamus
(44,
45). The synchronization of these oscillations at multiple cortical sites is likely to subserve a variety of mental processes such as perceptual organization of visual stimuli
(25–
27). Patients with schizophrenia show a deficit in their ability to use basic Gestalt principles for perceptual organization of visual stimuli
(17,
46). Most, but not all, studies have found that patients are relatively unable to benefit from the degree of perceptual organization in visual arrays
(47–
49). These findings are consistent with an abnormality in establishing cortical oscillations and a consequent inability to establish the synchronization of oscillations across cortical locations that is needed for the organization of visual arrays.
The possibility that patients have poor neural synchronization across sites is consistent with a recent hypothesis, mainly on the basis of theoretical grounds, that schizophrenia involves reduced cortico-cortical connectivity, leading to disruptions in feature binding
(50). This is also consistent with a formulation that emphasizes a breakdown in timing of cortical-subcortical connections, referred to as cognitive dysmetria
(51). Cortical-subcortical connectivity may be especially relevant to the possibility that cortical oscillations are driven by thalamic input
(44,
45). Neurocognitive measures of masking and perceptual organization may provide a means by which to operationalize notions of reduced neural connectivity or dysmetria in schizophrenia.
A limitation of the current study is that we did not have concurrent measures of gamma frequency activity. More direct indices of cortical oscillations in schizophrenia have started to come from recent electrophysiological studies. One study used neuromagnetic measures of the gamma response and suggested that aberrant cortical oscillations may explain poor suppression of the P50 response in schizophrenia
(28). An abnormality in establishing cortical oscillations could also explain findings from an EEG study by Hoffman et al.
(52). This study considered the dimensional complexity of EEG waveforms in neuroleptic-free patients with schizophrenia. Dimensional complexity reflects the number of independent variables contributing to the waveform and is considered an index of the complexity of cortical processing. The study evaluated the power spectrum of patients and comparison subjects, including the gamma range (31–50 Hz), during the performance of an activation task (a continuous-performance task). In this study, patients showed both reduced power in the gamma range and reduced EEG dimensional complexity. Results from both studies are consistent with a relative failure by patients to establish oscillations in the gamma range. Designers of future studies should consider combining performance measures (such as visual masking) with some of these more direct electrophysiological methods to assess gamma-range activity.
Backward masking procedures offer one example of how developments in cognitive neuroscience can be applied to questions in psychopathology. This study provides converging evidence that backward masking performance is a promising indicator of vulnerability to schizophrenia. In addition, the pattern of masking performance in patients is consistent with an abnormality in cortical oscillations in the gamma frequency. Although it is now speculative, aberrant cortical oscillations could yield a parsimonious account not only of visual masking deficits but of a variety of seemingly unrelated perceptual deficits in schizophrenia.