Patients with schizophrenia show deficits not only at higher cognitive but also at perceptual levels of processing. In the visual system, perceptual deficits have been repeatedly demonstrated in behavioral and electrophysiological studies using motion perception, luminance, and chromatic contrast detection, and visual backward masking tasks (see reference
1 for a review). However, it is unclear whether such deficits reflect early-stage versus top-down processing deficits. To examine substrates of visual processing dysfunction in schizophrenia, we used diffusion tensor imaging to evaluate white matter integrity at several levels of the visual system. The use of diffusion tensor imaging provides information about the structural basis for visual processing dysfunction.
Diffusion tensor imaging provides information about white matter integrity by using properties of water diffusion in brain tissue
(2) . In white matter, diffusion of water is greater in the direction parallel—rather than perpendicular—to axonal tracts and is termed “anisotropic.” Fractional anisotropy is a normalized measure of diffusion anisotropy and ranges from 0 (completely random) to 1 (completely unidirectional). In general, water molecules will be more restricted and fractional anisotropy will be higher, with greater white matter integrity.
Reduced diffusion anisotropy has been found in a number of areas, including the occipital white matter in schizophrenia
(3 –
5) . However, no study has systematically evaluated white matter integrity at different levels of the visual system. In the visual system, retinal input to the lateral geniculate nucleus is conveyed to the primary visual cortex (striate cortex) by means of optic radiations. The striate cortex projects to both the dorsal stream (“where system”) regions, such as inferior parietal lobule, and ventral stream (“what system”) regions, such as the fusiform gyrus. The placement of regions of interest in the optic radiations, striate cortex, inferior parietal lobule, and fusiform gyrus allowed white matter integrity to be investigated at different levels of the visual system.
Method
Informed consent was obtained from 17 patients (13 men and four women) who met DSM-IV diagnoses of schizophrenia (N=15) or schizoaffective disorder (N=2) and 21 healthy volunteers (13 men and eight women) after a full explanation of procedures. Diagnoses were obtained with the Structured Clinical Interview for DSM-IV and all available clinical information. All patients were receiving antipsychotic medications at the time of imaging (atypical or typical plus atypical). The Brief Psychiatric Rating Scale total score was 30.9 (SD=4.6).
The patient and comparison groups did not differ significantly in age (patients: mean=36.6, SD=11.0 years; comparison subjects: mean=34.9, SD=10.6 years) or gender ratio (p=0.49, Fisher’s exact test), although socioeconomic status, as measured by the four-factor Hollingshead-Redlich scale, was significantly lower for the patients (mean=23.8, SD=7.8) than for the comparison subjects (mean=45.5, SD=11.2) (t=6.7, df=36, p<0.001). Exclusion criteria were similar to those used by Ardekani et al.
(5) .
Imaging was conducted on a 1.5-T Siemens Vision system (Erlangen, Germany) at the Nathan Kline Institute, as previously described
(5) . Briefly, axial diffusion tensor imaging scans were acquired with a pulsed gradient, double spin echo, echo-planar imaging sequence (TR/TE=6000/100 msec, 128×128 matrix interpolated to 256×256, field of view=240 mm, b=900 s/mm
2, number of excitations=four, 20 slices, 5-mm slice thickness, 0-mm gap, six diffusion directions). Fractional anisotropy measures were calculated as described by Basser et al.
(2) and Hoptman et al.
(6) . Image analysis and quantification were performed by using a custom system based on Interactive Data Language software (Research Systems, Boulder, Colo.).
Regions of interest were placed bilaterally in four different regions: 1) optic radiations (slice corresponding to the anterior commissure-posterior commissure plane immediately adjacent to the trigone of the lateral ventricle), 2) the striate cortex (~15 mm above the anterior commissure-posterior commissure plane in white matter in the most posterior regions of the occipital cortex), 3) the inferior parietal lobule (25 mm above the anterior commissure/posterior commissure plane), and 4) the fusiform gyrus (~10 mm below the anterior commissure/posterior commissure plane). Regions of interest of the same size for each brain area were drawn separately for each participant on the T-weighted (b=0) images
(6) . It should be noted that the striate cortex region of interest may include not only V1 but also V2. Interrater reliability showed intraclass correlations between two raters (P.D.B. and M.J.H.) of 0.77–0.95 for all for the regions of interest. Separate two-by-two analyses of variance (ANOVAs) with group and hemisphere as factors were performed for each brain area.
Results
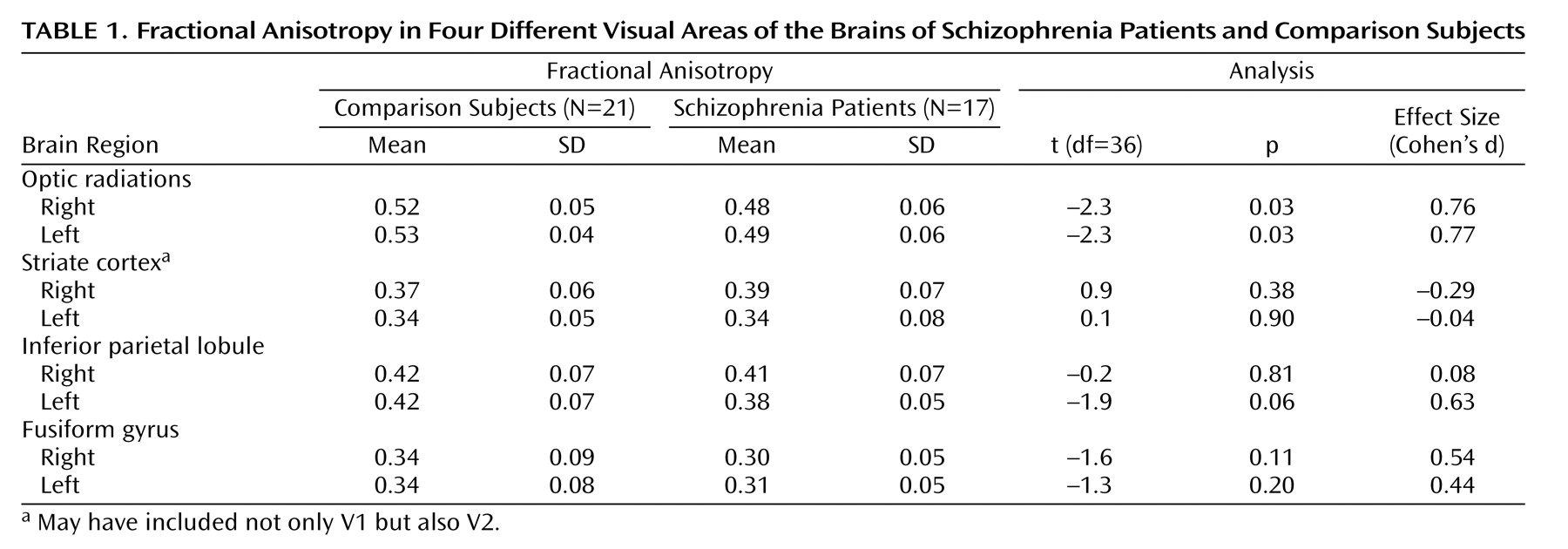
For the optic radiations, ANOVAs showed significant between-group effects for patients versus comparison subjects (F=10.03, df=1, 36, p=0.003), with no significant interaction of group and hemisphere (F=0.10, df=1, 36, p=0.75). Right and left optic radiation fractional anisotropy were decreased by 8.1% and 6.6%, respectively (
Table 1 ).
Separate ANOVAs for the inferior parietal lobule, the striate cortex, and the fusiform gyrus did not show any significant between-group effects or group-by-hemisphere interactions, with the exception of a tendency for a significant between-group effect for the fusiform gyrus (F=3.16, df=1, 36, p=0.08).
Discussion
This study systematically traced white matter integrity from primary visual sensory to association areas. Fractional anisotropy was decreased in the optic radiations, which project from the lateral geniculate nucleus to the striate cortex, but not in the striate cortex or higher-level striate projection areas. These data suggest that integrity of the earliest pathway is most affected.
The optic radiations carry information from two main pathways: the magnocellular pathway, which has large axons and is rapidly conducting, and the parvocellular pathway, which has smaller axons and is more slowly conducting. From the striate cortex, the magnocellular pathway projects mainly to the dorsal stream and the parvocellular pathway to the ventral stream. Although we and others have demonstrated preferential magnocellular dysfunction in schizophrenia, parvocellular deficits occur as well (for a review, see reference 1). Decreased fractional anisotropy in the optic radiations is consistent with both magnocellular and parvocellular dysfunction. Greater deficits in magnocellular function could be due to pathology in the larger caliber magnocellular pathway. Although the optic radiations are the main tract in the region of interest, it cannot be ruled out that other fibers may contribute to fractional anisotropy in this region.
This is the first study, to our knowledge, that has examined white matter integrity in the striate cortex or the fusiform gyrus. No significant white matter changes, assessed with fractional anisotropy, were found in these areas, although an ANOVA showed a tendency for a decrease in the fusiform gyrus. A previous study found decreased fractional anisotropy in the inferior parietal lobule
(5) . We found a tendency toward a decrease in left hemisphere fractional anisotropy in the inferior parietal lobule, but this was not significant. Gray matter deficits have been found in the striate cortex, the inferior parietal lobule, and the fusiform gyrus
(7 –
9), which supports the concept that structural deficits intrinsic to visual areas in schizophrenia underlie known perceptual deficits. Nonsignificant decreases in fractional anisotropy in the fusiform gyrus and the parietal lobe were also observed, suggesting possible secondary deficits in these regions as well.
In conclusion, fractional anisotropy measures show significant white matter deficits in the optic radiations. The robust bilateral decrease in white matter integrity in optic radiations provides a structural basis for the hypothesis that visual processing deficits in schizophrenia occur at the early stages of processing. Such early-stage deficits may contribute to higher-level perceptual deficits.