Theoretical accounts of the core symptoms and cognitive problems in attention deficit hyperactivity disorder (ADHD) nearly all address aspects of self-regulation in some form
(1) . Self-regulation—or
cognitive control, in contemporary terms—is characterized as the ability to suppress inappropriate actions in favor of appropriate ones. This issue of
The American Journal of Psychiatry includes three articles that report unique patterns of brain activity in ADHD using functional magnetic resonance imaging and versions of cognitive control tasks across different ages. Examples of cognitive control in these studies are shown in instances where overriding an action to an expected target in favor of an unexpected nontarget in an oddball or go/no-go paradigm occurs—reported in this issue of the
Journal by Smith and colleagues and Pliszka and colleagues—or when switching attention from one salient stimulus attribute (color) to another (shape) occurs—reported in this issue of the
Journal by Tamm and colleagues. These studies underscore the importance of tracking developmental changes in cognitive processes implicated in ADHD, acknowledging individual differences in ADHD, and moving away from empirical studies to more theoretical and testable models of the disorder.
Identification of core processes involved in a disorder can move a field from a disparate set of data-driven findings to a more theoretically coherent collection of studies. Barkley’s account of ADHD perhaps best illustrates this point. Barkley suggested that cognitive deficits observed in ADHD may be secondary to a single deficit in
inhibitory control, where deficits in working memory, for example, are due to an inability to suppress reactions to distracting information
(2) . Although it has been argued that this simple explanation cannot explain all the phenotypic variance in ADHD, this view emphasizes one important point: that cognitive (and neural) processes are intrinsically linked, and thus deficits in one system are likely to affect others in secondary ways, especially in a dynamically changing system, such as the developing child with ADHD. Functional imaging studies, such as the ones reported in this issue of the
Journal, rely on the specificity and experimental design of the cognitive tasks individuals perform while brain activity is imaged. Thus, the only way to measure the underlying neural systems involved in ADHD is if the selected tasks truly capture the behavioral manifestations of the disorder. As such, imaging studies will always be task-specific, and only with theoretical guidance will findings from these kinds of studies be constrained. Smith and colleagues, in part, address this issue by reporting imaging results not just from one but from three cognitive control tasks.
Functional imaging studies of ADHD to date have focused largely on top-down cortical control systems (e.g., the prefrontal cortex) in trying to understand the breakdown in regulating actions and biasing of attention in favor of relevant information. The focus on prefrontal brain regions is based on the neuropsychological literature showing that frontal lobe damage impairs the ability to regulate behavior. Although this approach has been important in describing problems in cognitive control, it has not captured the biological basis of how self-regulation breaks down in ADHD. Individuals with ADHD do not have a specific brain lesion. In addition, it would not be expected for an insult in a developing dynamic system to have the same impact as an insult in a mature system.
Less attention has been given to bottom-up neural systems such as the basal ganglia and cerebellum
(3) or to posterior cortical systems implicated in ADHD, as exemplified in the study by Tamm and colleagues. At first glance, findings of multiple regions across several imaging studies would seem too disparate to be informative; however, considering these findings in the context of cognitive control theory, they paint a clearer picture. According to cognitive control theory, control should be implemented when something unexpected occurs in order to adjust behavior appropriately. Implicit in this notion is that the child with ADHD must be able to learn to predict future events in order to detect unpredicted ones. This ability is essential in planning and maintaining appropriate thoughts and actions in different social contexts over time. But what if these basic learning systems are compromised?
We have recently proposed a model of ADHD, based largely on computational and animal studies of learning, whereby basic learning systems are important in signaling top-down control systems to adjust behavior when predicted outcomes are violated
(1) . The basic assumption of this model is that learning when, what, or in which contexts to expect an event is critical for the ADHD child in adjusting behavior appropriately in different contexts. Deficits in learning to detect regularities or irregularities in the environment could lead to less signaling of control systems to help alter or adjust behavior accordingly. Such deficits could mimic those observed when top-down control systems themselves are impaired.
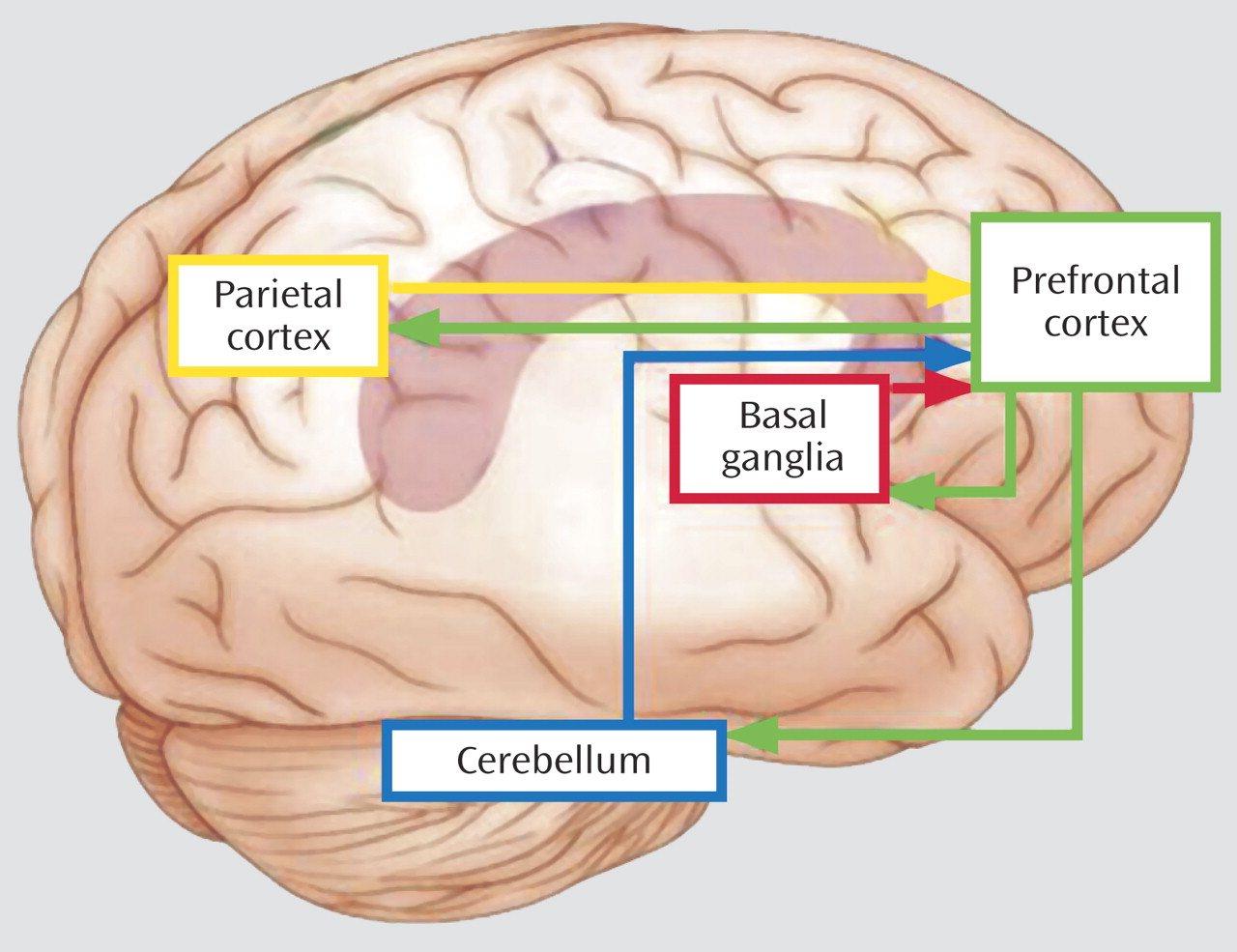
Figure 1 depicts brain regions activated across the three imaging studies in this issue of the
Journal . The basal ganglia and cerebellum have been implicated in learning about the frequency and the timing of events (i.e., learning what to expect and when), and the posterior parietal region has been implicated in signaling prefrontal cortex when competing stimuli require top-down biasing of attention in favor of one stimulus attribute or location over another. Each of these regions (basal ganglia, cerebellum, and parietal cortex) is part of unique circuits that project both to and from prefrontal cortex, thus providing a means for signaling prefrontal regions to help impose top-down control of behavior. Ineffective signaling of control systems by any one of these regions could lead to poor regulation of behavior but with subtle differences, depending on the system impacted and the task used. Likewise, intact signaling but inefficient top-down control could result in poor regulation of behavior but, presumably, in a more general way. Variability in cognitive performance reported in ADHD literature may be explained by such differences. Cognitive development has been characterized as the growing ability to filter and suppress irrelevant information and actions in favor of more relevant ones
(4) . Developmental changes in prefrontal circuitry most likely contribute to the emergence of this ability
(5) . Therefore, development must be considered in delineating the contributions of different brain regions (e.g., the prefrontal cortex, striatum, cerebellum, parietal cortex) to cognitive control and its disruption in ADHD.
The imaging studies of ADHD in this issue of the
Journal cover ages 9 to 27 years, with limited ability to track changes in neural systems over the course of this age range. Given that cognitive abilities most affected in ADHD show large developmental and individual differences, it may seem perplexing that functional imaging studies of ADHD have not emphasized tracking changes longitudinally within individuals. Yet those of us who engage in imaging research recognize the hurdles of such an approach with individuals with ADHD. The primary difficulty lies in the susceptibility of imaging methods to movement and the tendency of this population to move, especially at younger ages
(6) . Nonetheless, examining the longitudinal progression of ADHD within individuals would further delimit our understanding of the cognitive and neural processes underlying the disorder.
How do the imaging studies of ADHD in this issue of the
Journal add to the growing literature in this area, and how do they inform the field in terms of biological substrates, treatment, or intervention? Perhaps the most compelling finding is that stimulant use does not appear to explain the different patterns of activity observed between individuals with and without ADHD, as reported by Pliszka and colleagues and Smith and colleagues. Although the authors report differences in prefrontal activation between children with ADHD and comparison subjects, they find very few differences between children with ADHD who had previously taken stimulants and those who had not. These findings are consistent with magnetic resonance imaging-based anatomical findings of minimal differences in brain structure between previously treated and stimulant-naive children with ADHD
(7) . However, acute administration of methylphenidate alters brain activity, especially in the striatum, and simultaneously improves symptoms in children with ADHD
(8) . Positron emission tomography studies support these findings by showing that stimulant medication works by blocking the dopamine transporter, particularly in the striatum
(9) . These types of neuroimaging studies have the potential to disentangle long- and short-term effects of medication from changes associated with the disorder itself.
Perhaps the most exciting new direction for imaging studies of ADHD, beyond the functional and pharmacological studies described, is the recent emergence of genetic imaging (i.e., imaging individuals with a particular genotype relative to another). Heritability studies and the large variability in cognitive performance and symptoms observed across individuals with ADHD suggest different pathways to the disorder, including both genetic and environmental pathways (e.g., CNS-insults). Examining genes that are expressed in brain regions implicated in ADHD
(10) may be informative as to which treatments will be most effective for which variant of the disorder and ultimately lead to individualized treatment. In summary, imaging studies have helped us understand the cognitive and neural processes underlying ADHD, but recognizing the phenotypic variability in symptoms and behavior will be essential in optimizing treatment for individuals with this disorder.