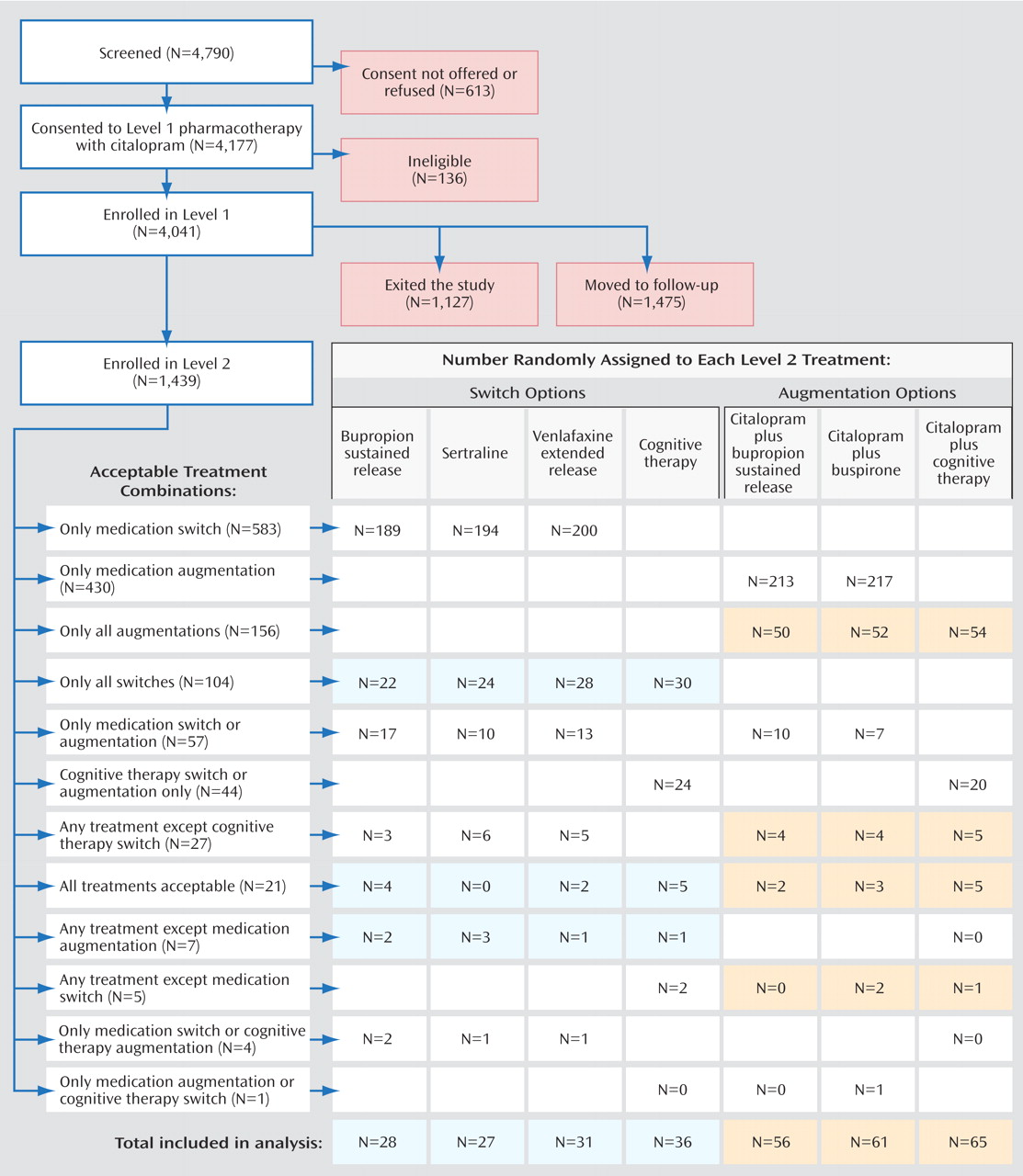
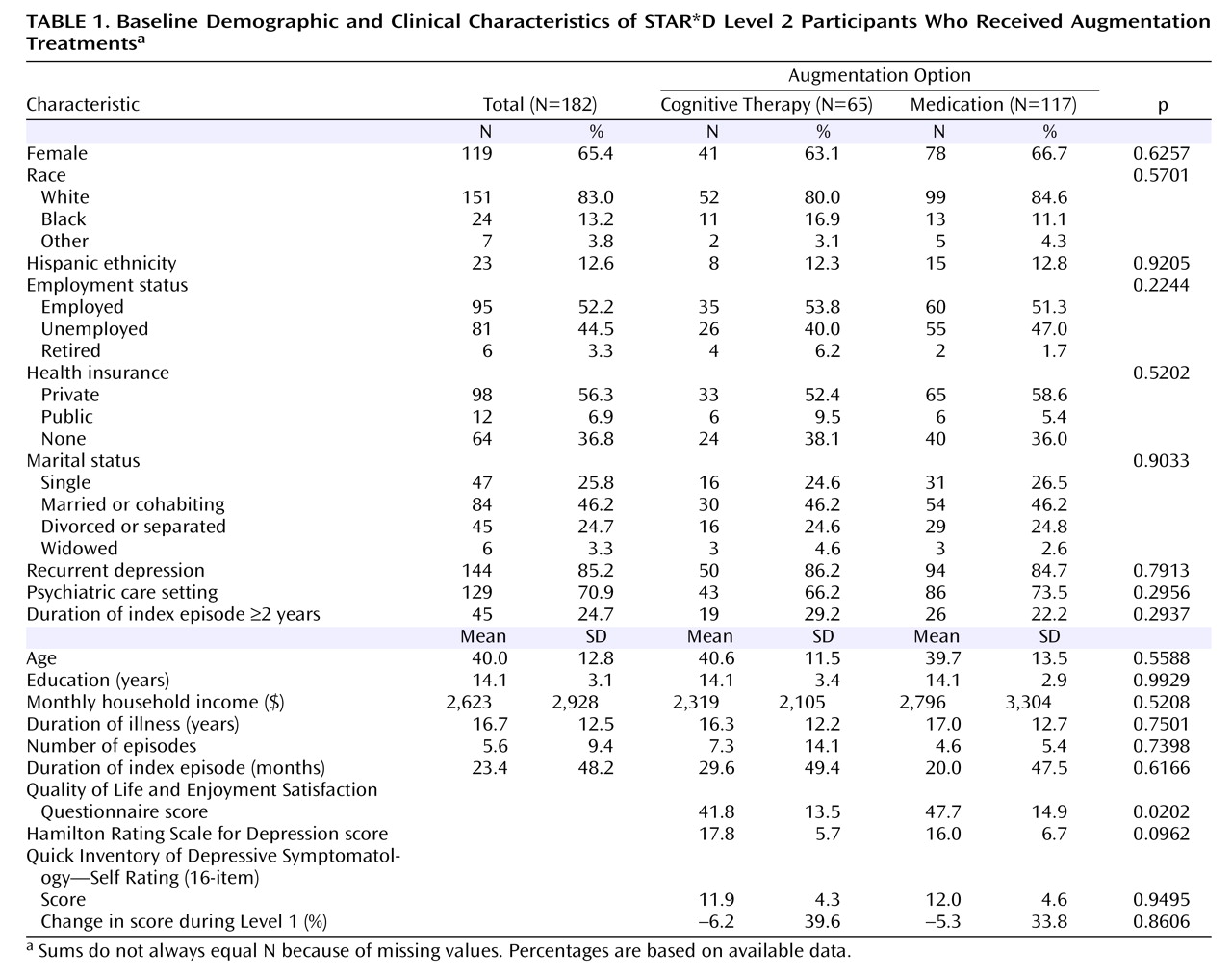
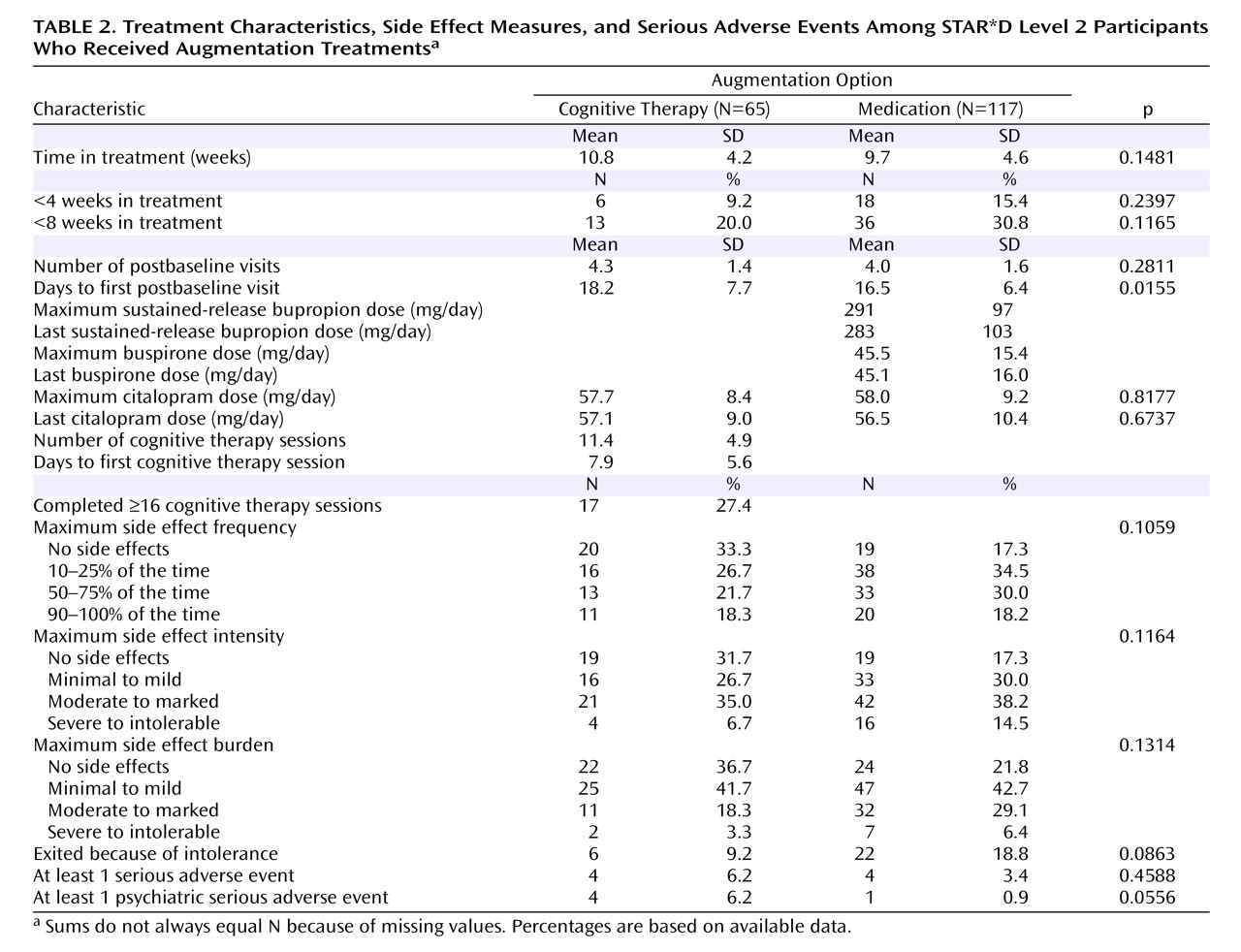
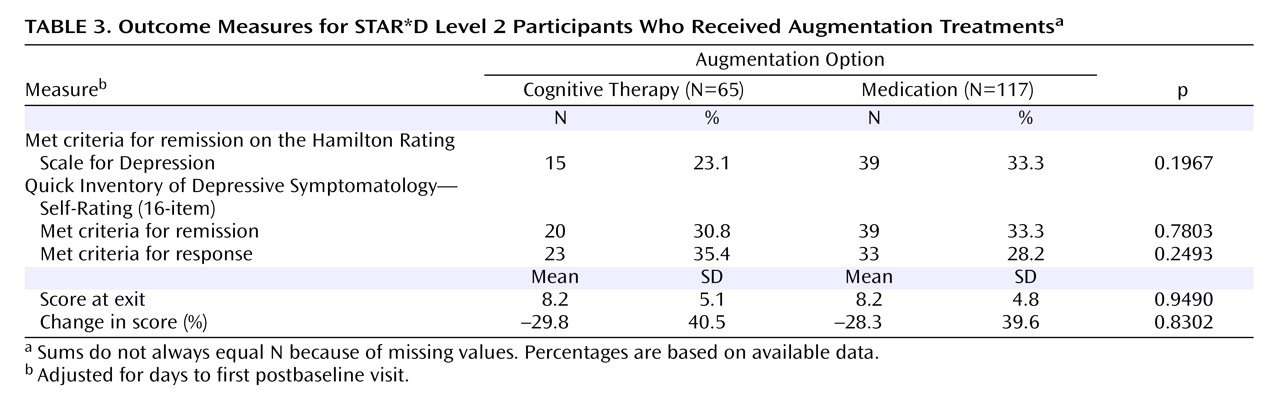
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial is a multicenter, multistage research project funded by the National Institute of Mental Health with the specific purpose of evaluating second-, third-, and fourth-step treatment options for patients with treatment-resistant depression
(7,
8) . In the first treatment step (up to 14 weeks with citalopram alone), approximately one-third of 2,876 participants remitted
(9) . In a previous report on second-step treatments
(10), we compared switching from citalopram to alternative second-step antidepressants. There were no significant differences in outcome, with intent-to-treat remission rates ranging from 18% with sertraline (a selective serotonin reuptake inhibitor) to 21% with sustained-release bupropion (a norepinephrine-dopamine reuptake inhibitor) to 25% with extended-release venlafaxine (a serotonin-norepinephrine reuptake inhibitor). A second report compared sustained-release bupropion and buspirone as augmentation agents and found few differences in effectiveness, with both treatments resulting in intent-to-treat remission rates of 30%
(11) .
We now report on the utility of cognitive therapy following nonremission with, or intolerance to, citalopram as compared with pharmacologic augmentation and switch strategies. Cognitive therapy is the best-studied form of psychotherapy for acute therapy of major depressive disorder
(1,
12) and has been proposed to be a useful alternative for patients who have not responded to antidepressant medications
(13 –
15) . A number of case series and small randomized trials suggest that cognitive therapy may indeed have a role in the management of treatment-resistant depression
(16) . The largest study to date
(17) found that a form of cognitive behavior therapy was at least as effective when used as a second-step treatment after nonresponse to nefazodone as nefazodone was after nonresponse to the psychotherapy. However, no large-scale randomized studies have evaluated the utility of second-step psychotherapy as compared with medication interventions.
Method
The rationale and design of STAR*D and the specific elements of the cognitive therapy protocol have been detailed elsewhere
(7 –
11,
18) . A summary of the study design is presented below.
Participants
From July 2001 through April 2004, STAR*D enrolled 4,041 outpatients 18 to 75 years of age with a diagnosis of nonpsychotic major depressive disorder from 18 primary care and 23 psychiatric care practice settings across the United States. The diagnosis was clinically established and verified by a checklist based on DSM-IV criteria
(19) . Advertising for participants was proscribed to ensure that recruitment would produce a sample representative of patients seen in typical clinical practice. Written informed consent was obtained at study entry and again at enrollment in the second-step treatments.
Inclusion and Exclusion Criteria
STAR*D used broad inclusion and minimal exclusion criteria
(7,
8) to ensure enrollment of a representative study sample of depressed patients seeking treatment in primary care, public mental health, and psychiatric clinics. For example, most forms of comorbidity commonly associated with depression were permitted. The minimum score on the 17-item Hamilton Rating Scale for Depression (HAM-D)
(20,
21) for enrollment was 14, indicating at least a moderate level of depression.
Treatment Protocol and Therapist Training
To ensure rigorous dosing, reflect actual practice, and enhance safety, treatments were not masked to participants or providers. The primary outcome measure was whether participants achieved symptom remission, defined as a score ≤7 on the HAM-D, which was administered by research outcome assessors who were blind to treatment assignments. The protocol recommended that medication treatment visits for all treatment levels be conducted at weeks 0, 2, 4, 6, 9, and 12; however, the visit schedule was flexible, and extra visits could be held if clinically indicated. Depressive symptom severity was assessed at each treatment visit using the clinician-administered version of the 16-item Quick Inventory of Depressive Symptomatology (QIDS-C)
(22 –
24) . If participants had a response (defined as a reduction of ≥50% in baseline QIDS-C score) without remission at week 12, they could continue treatment for an additional 2 weeks (14 weeks total) to determine whether remission would occur with additional time. Remission at the clinic visits was defined as a QIDS-C score ≤5. The frequency, intensity, and burden of side effects were monitored with a scale developed for the STAR*D study
(25) .
To increase the likelihood that each patient received an adequate course of pharmacotherapy, each STAR*D site had a clinical research coordinator who monitored the progress of each participant, treating physicians received in-service training based on a clinician manual, and a web-based medication monitoring system
(26) provided ongoing feedback on patients’ symptom ratings, side effect burden, and treatment regimen
(9) . The protocol for the initial course of pharmacotherapy in Level 1 of the STAR*D sequence called for starting citalopram at a dose of 20 mg/day, to be raised to 40 mg/day by week 4. In the case of nonresponse, the dose could be raised to 60 mg/day by week 6. All medication dose recommendations were flexible and could be applied on the basis of clinical judgment informed by scores on the side effect rating scale and the QIDS-C, which were obtained at treatment visits by researchers blind to the patients’ HAM-D score. In the case of intolerable side effects, patients could be withdrawn from Level 1 and advanced to the next treatment level. Patients who did not achieve remission with citalopram were encouraged to move to Level 2 after 12 weeks of citalopram therapy. Those who achieved remission could enter a 12-month naturalistic follow-up phase, and those who had a response without remission were strongly encouraged to proceed to Level 2, although they could also elect to enter the follow-up phase. The flow of patient progress and outcomes from Level 1 to Level 2 is summarized in
Figure 1 .
Level 2 Treatments
For patients who did not remit with or tolerate the initial course of citalopram therapy, there were seven possible second-step treatment options. The aims of the study included comparing three augmentation options (adding sustained-release bupropion, buspirone, or cognitive therapy to ongoing citalopram therapy) and four switch strategies (discontinuing citalopram and starting therapy with sertraline, sustained-release bupropion, extended-release venlafaxine, or cognitive therapy).
A novel element of the STAR*D research design, equipoise-stratified randomization
(27), enabled participants—in consultation with their treating physician—to eliminate the possibility of being randomly assigned to treatment strategies they found unacceptable and still remain in the study (thereby mirroring practice). For example, participants could elect to be randomly assigned only to the switch treatments or only the augmentation treatments. They could also accept or decline cognitive therapy within either of these strategies. Or they could exclude all treatments except cognitive therapy alone (as a switch) and cognitive therapy as an augmentation to citalopram. They were subsequently randomly assigned to receive one of the remaining treatment options. In each unique cluster of acceptable treatment options, participants were treated as a stratum in data analyses. The numbers of participants in each stratum provided an approximate ranking of the overall acceptability of both the broader strategies (i.e., augmentation versus switch) and selected treatments.
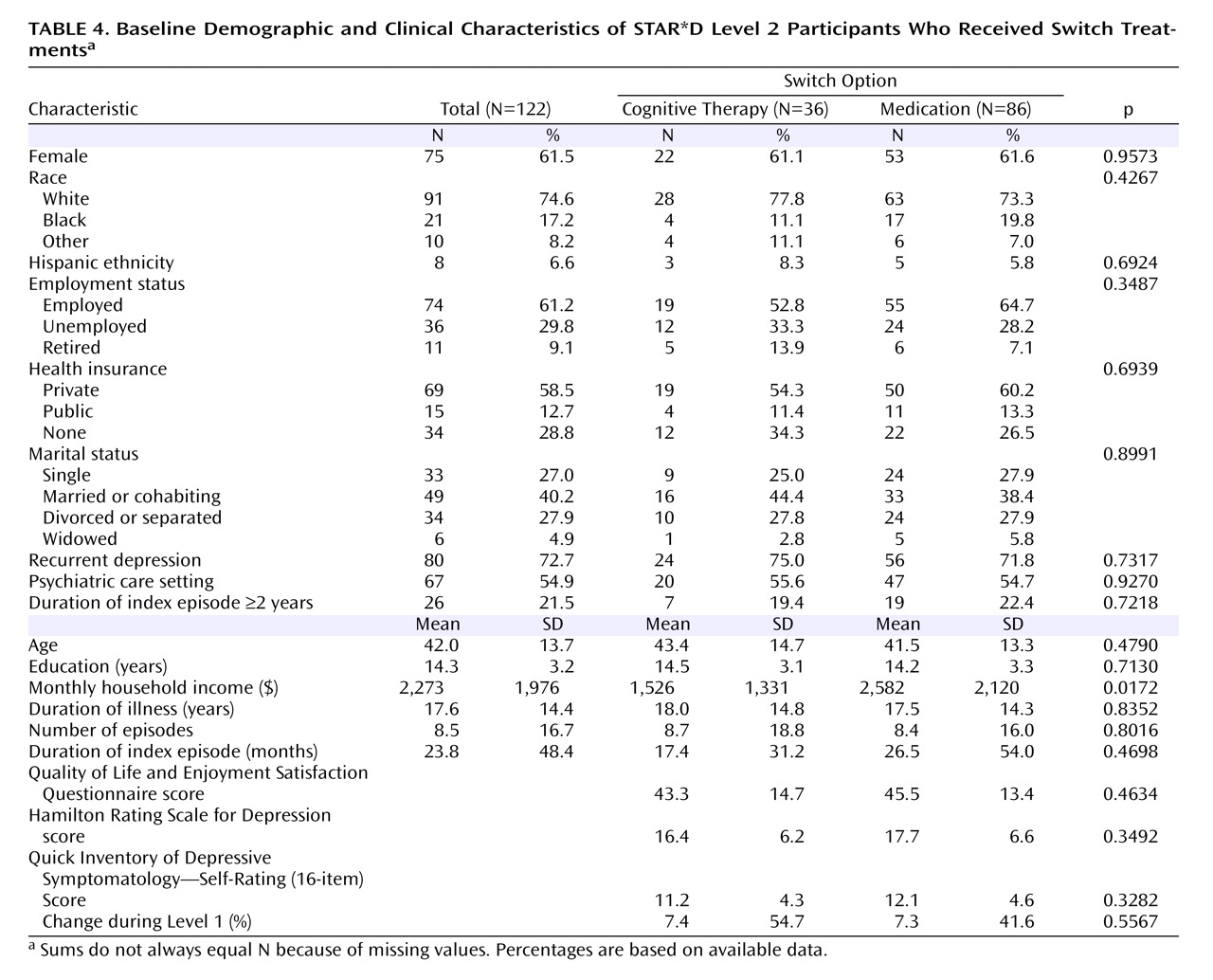
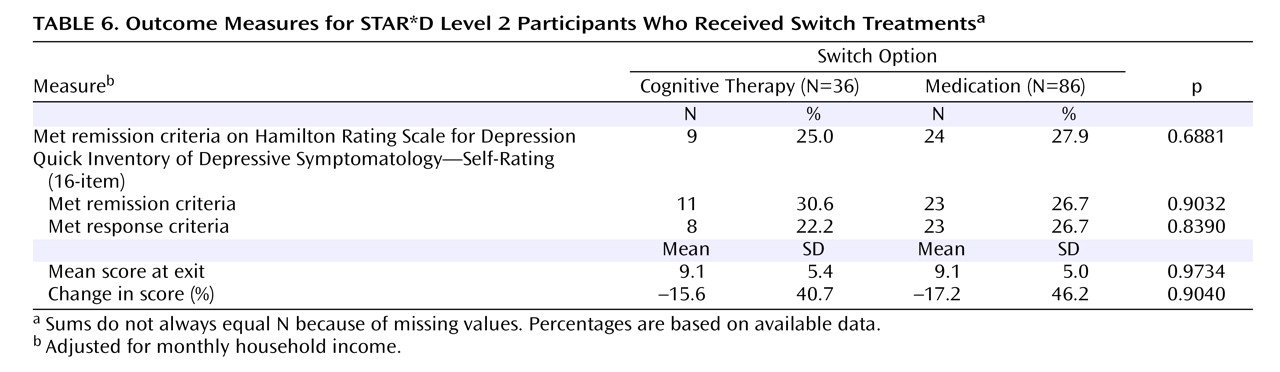
Of the 4,041 outpatients enrolled in STAR*D Level 1, 1,439 (36%) did not achieve a satisfactory response with citalopram and moved to Level 2. In theory, if all participants had accepted all seven treatment options, on average two out of seven who entered Level 2, or 411 patients (1,439×2/7; 29%) would have been assigned to cognitive therapy. In fact, only 369 participants (26%) accepted one or both cognitive therapy options, and only 147 were assigned to cognitive therapy in Level 2 (including 44 who elected to accept only cognitive therapy options, either alone or as an augmentation to citalopram). Of the 752 patients who were willing to accept an augmentation strategy, 209 (28%) were willing to accept both cognitive therapy and pharmacotherapy among the options; 182 of them were randomly assigned to augmentation of citalopram with either cognitive therapy (N=65) or another medication (N=117). Among the 853 patients who were willing to accept a switch strategy, 132 (16%) were willing to accept both cognitive therapy and pharmacotherapy among the options; 122 of them were randomly assigned to either cognitive therapy alone (N=36) or a second course of pharmacotherapy (N=86). Across the switch and augmentation strata, there was essentially no difference in the acceptability of receiving cognitive therapy among patients treated at primary care sites and in psychiatric settings (25.4% and 25.8%, respectively).
Cognitive Therapy
To ensure that results were generalizable to clinical settings, selection and training of therapists mimicked good clinical practice. Each site’s primary investigator proposed candidate practicing psychotherapists for the study. Candidate therapists submitted summaries of their relevant training and experience and video- or audiotapes of their work with depressed patients. Only one candidate was not accepted for further training, although a number of other candidates did not complete training. Candidate therapists received relevant readings and attended a 2-day workshop conducted by study investigators (E.S.F. and M.E.T.). Training followed the approach described by Shaw
(28), which had been used by the cognitive therapy research group at the University of Pittsburgh for more than 15 years. Before they could participate in the study, therapists had to demonstrate competence (documented by fidelity ratings on the Cognitive Therapy Scale
[18] ) in the treatment of one patient who would have been eligible to participate in STAR*D. A total of 44 therapists (30 doctoral-level psychologists, one physician, 11 master’s-level clinical social workers, and two nurses with advanced degrees) completed the certification process; each site had at least two certified therapists. Each therapist treated an average of 2.1 study patients (range=1–6 patients per therapist).
During the study, the psychotherapists’ patients were monitored for adequate therapeutic response using a web-based system that was updated weekly. During monthly group supervision sessions, case conceptualizations, problems implementing cognitive therapy, and treatment strategies were reviewed in detail. If therapists experienced significant problems during the course of the study, additional sessions of individual supervision were provided. No therapists were withdrawn from study participation because of nonadherence to the cognitive therapy model
(18) .
The protocol required that cognitive therapy be scheduled twice weekly for weeks 1–4, then once weekly for the remaining 8 weeks (16 sessions total). When twice-weekly sessions were not practicable, the second session could be conducted by telephone. Patients who improved rapidly, as defined by at least three consecutive weeks of remission, could enter follow-up without completing all 16 visits. Patients who had a response but not remission by visit 16 could continue to Level 3 treatment or elect to continue cognitive therapy for an additional four weekly sessions, then twice a month for an additional four sessions and monthly for the final six sessions (research has shown that such patients benefit from continuation-phase cognitive therapy
[29] ).
Pharmacotherapy
Pharmacotherapy visits during Level 2 typically lasted 15–20 minutes. Although pharmacotherapists were not prevented from using psychotherapeutic strategies, it is unlikely that much in the way of psychotherapy was provided in these sessions beyond supportive encouragement and psychoeducation. For patients assigned to the augmentation options, the dose of citalopram was typically kept steady, although it could be reduced as needed to alleviate side effects. The target dosing for sustained-release bupropion was 200 mg/day for weeks 1–2, to be raised to 300 mg/day by week 4 and to 400 mg/day as a final dose. Buspirone dosing was to start at 15 mg/day for 1 week, to be raised to 30 mg/day for 1–2 weeks and then to 45 mg/day by week 4, with a maximum dose of 60 mg/day after week 6. For the switch options, citalopram was discontinued at the initial visit, and the new treatment was begun without a tapering or washout period. Sustained-release bupropion dosing was to start at 150 mg/day for 1 week, to be raised to 300 mg/day thereafter, with 400 mg/day as a final dose. Sertraline dosing was to start at 50 mg/day for 1 week, to be raised to 100 mg/day for weeks 3–4, to 150 mg/day for weeks 5–9, and to 200 mg/day for weeks 10–12. The starting dose for extended-release venlafaxine was 37.5 mg/day for 3 days, to be raised to 75 mg/day for week 2, to 150 mg/day for weeks 3–4, to 225 mg/day for weeks 5–6, to 300 mg/day for weeks 7–9, and to 375 mg/day for weeks 10–12.
Measures
At study intake, clinical research coordinators collected standard sociodemographic information and self-reported psychiatric history (including an assessment of suicidality) and completed the HAM-D and QIDS-C, as well as the Cumulative Illness Rating Scale
(30,
31) to measure medical comorbidity. The self-report Psychiatric Diagnostic Screening Questionnaire
(32) was administered to assess for the presence of 11 concurrent psychiatric disorders
(33) .
For the primary outcome measure, the HAM-D was administered in telephone interviews conducted by the research outcome assessors at entry and exit from each treatment level. Secondary outcomes measures obtained by the research outcome assessors included the 30-item Inventory of Depressive Symptomatology—Clinician Rating
(34 –
36), as well as the 5-item Income and Public Assistance Questionnaire to measure monthly income by source. Anxious depression was defined on the basis of pretreatment scores on the anxiety/somatization factor of the HAM-D
(37) . Items from the Inventory of Depressive Symptomatology were used to establish the presence or absence of atypical features
(38) and melancholic features
(39) . Research outcome assessors blind to treatment assignments repeated the HAM-D, Inventory of Depressive Symptomatology, and Income and Public Assistance Questionnaire at the end of each treatment level.
A telephone-based interactive voice response system
(40,
41) was used to collect function and quality-of-life measures from participants within 72 hours of study entry and exit of each level, including the 16-item Quality of Life Enjoyment and Satisfaction Questionnaire
(42) to assess quality of life, the 12-item Short Form Health Survey
(43) to evaluate perceptions of mental and physical function, and the Work and Social Adjustment Scale
(44) to measure occupational and interpersonal impairment. The interactive voice response system was also used to administer the self-report version of the QIDS (QIDS-SR)
(22 –
24) for assessment of depression symptom severity at study entry, at week 6, and at exit from each level. Secondary symptom outcome measures included response and remission rates defined a priori by the QIDS-SR score. Remission was defined as a QIDS-SR score ≤5; response was defined as a reduction of ≥50% from baseline QIDS-SR score.
Statistical Analyses
Summary statistics are presented in the form of means and standard deviations for continuous variables and counts and percentages for discrete variables. Parametric and nonparametric analysis of variance methods and chi-square tests were used to compare baseline clinical and demographic features, treatment options, side effect measures, and serious adverse event rates across treatment group and for the entire sample.
All analyses were conducted using all patients in each randomization group
(45) . Separate analyses were performed for the augmentation and switch strategies. The primary outcome measure was the intent-to-treat HAM-D remission rate at study exit. Participants for whom outcome HAM-D scores were missing were assumed not to have achieved remission
(8) . Secondary outcome measures included response and remission rates at study exit according to the QIDS-SR. Adjustments for potential confounding effects were limited because of sample size and the inability of the models to converge. Exact logistic regression models were used to compare remission and response rates after adjusting for significant between-group differences (the effect of days to first postbaseline visit for the comparison of augmentation strategies and income in the comparison of switch strategies). To determine whether cognitive therapy had a differential effect by practice setting, exact logistic regression models were fit, including main effects for treatment and setting, as well as the two-way interaction. Because assessment with the QIDS-SR was more frequent across the treatment protocol, this measure was used to estimate the rapidity of response. Times to first remission (QIDS-SR score ≤5) and first response (≥50% reduction from baseline QIDS-SR score) were defined as the first observed point using clinic visit data. Survival hazard functions were estimated using the Kaplan-Meier method, and log-rank tests were used to compare the cumulative proportions of remission and response among the treatment groups.
All Level 2 comparisons in STAR*D were planned to have at least 80% power to detect between-group differences in remission rates of ≥15% (assuming approximately 200 patients per arm, use of two-tailed tests, and an alpha level of 0.05). Because the numbers of consenting participants in the cognitive therapy arms were substantially smaller than planned, the study had 80% power to detect only larger between-group differences, on the order of 22.5% for the augmentation arm and 29% for the switch arm.
Discussion
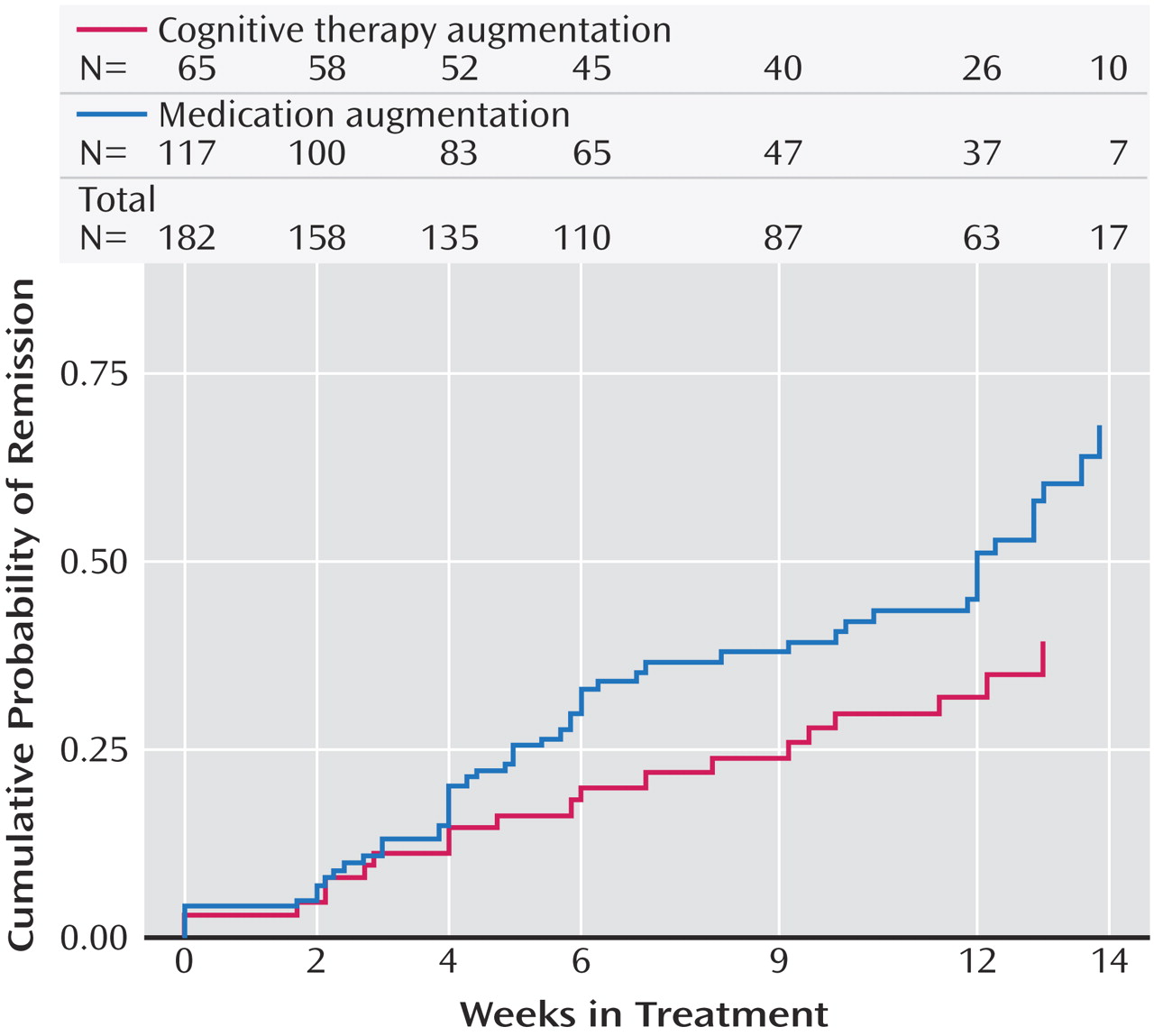
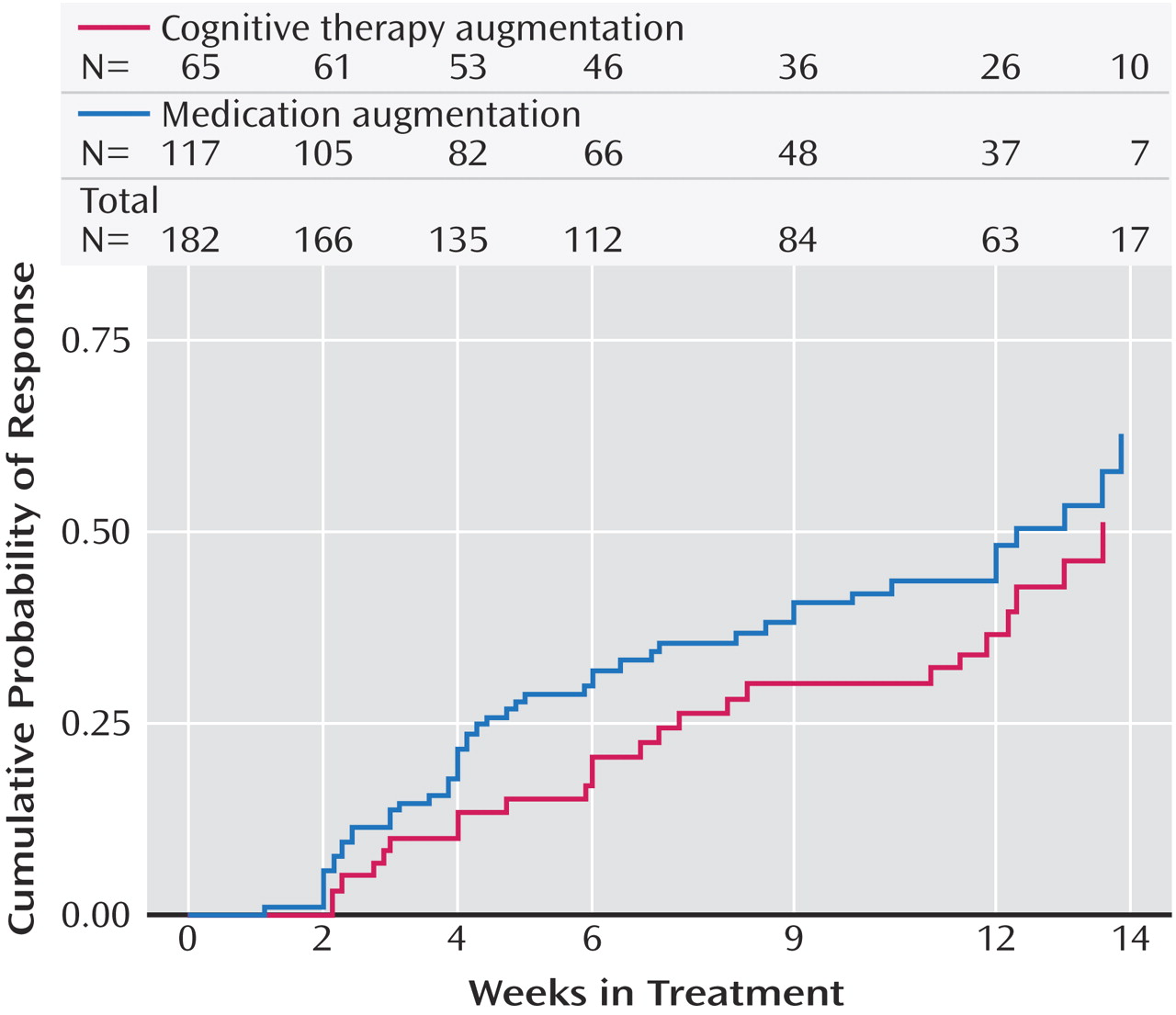
Perhaps the most important finding of this study was that cognitive therapy, both alone and in combination with citalopram, was generally as effective as the various second-step pharmacologic strategies studied in STAR*D. Among participants who opted for an augmentation strategy, the addition of cognitive therapy ultimately resulted in about the same probability of remission and a similar degree of symptomatic improvement as adding sustained-release bupropion or buspirone. The benefit of cognitive therapy was slower to emerge, however, with a significant 20-day difference in median time to remission favoring pharmacologic augmentation. When speed of response is imperative, this 3-week advantage could be of considerable importance. An unanticipated finding was that pharmacologic augmentation was nearly as well tolerated as cognitive therapy augmentation. In fact, none of the between-group differences in side effect frequency, intensity, or burden were statistically significant.
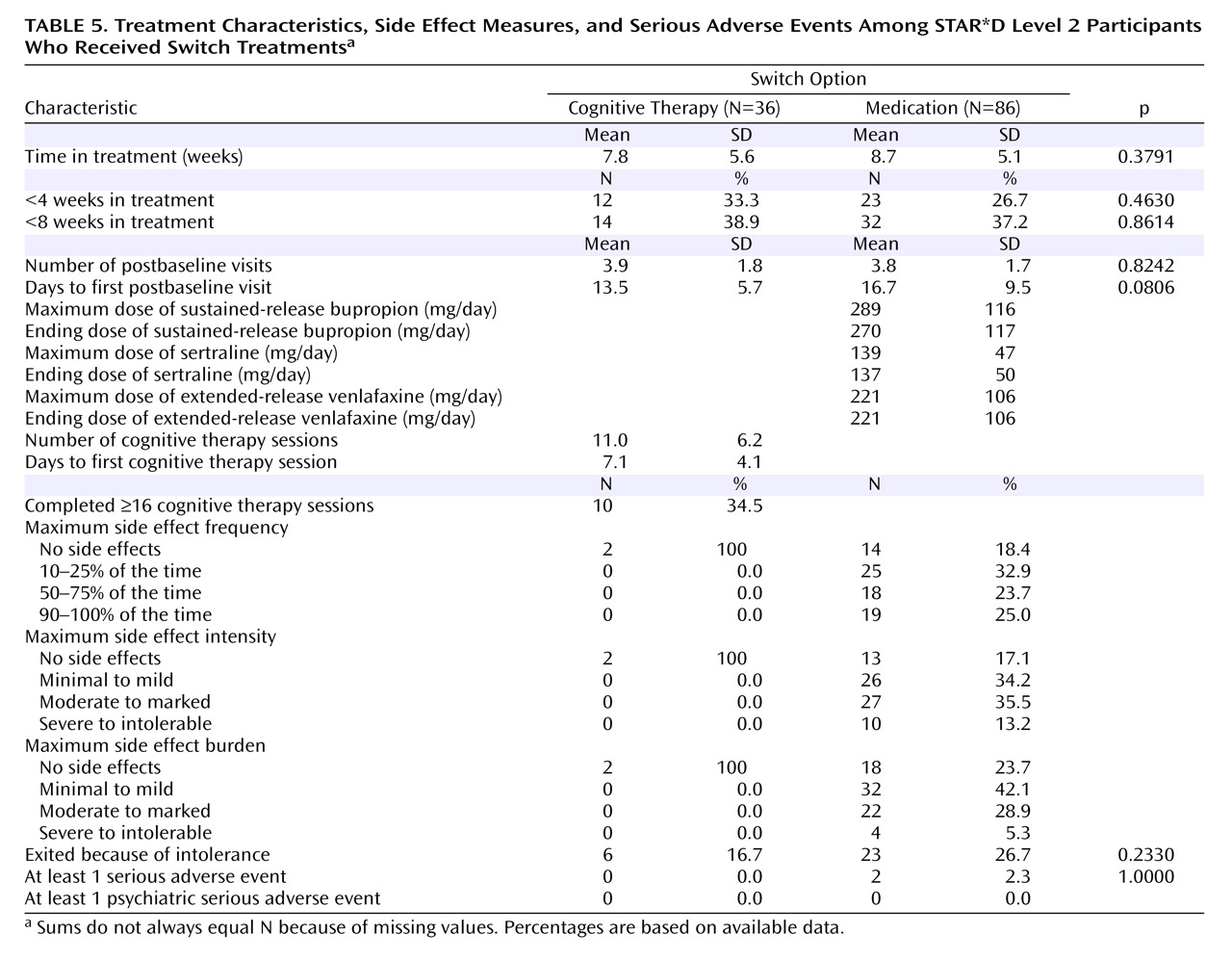
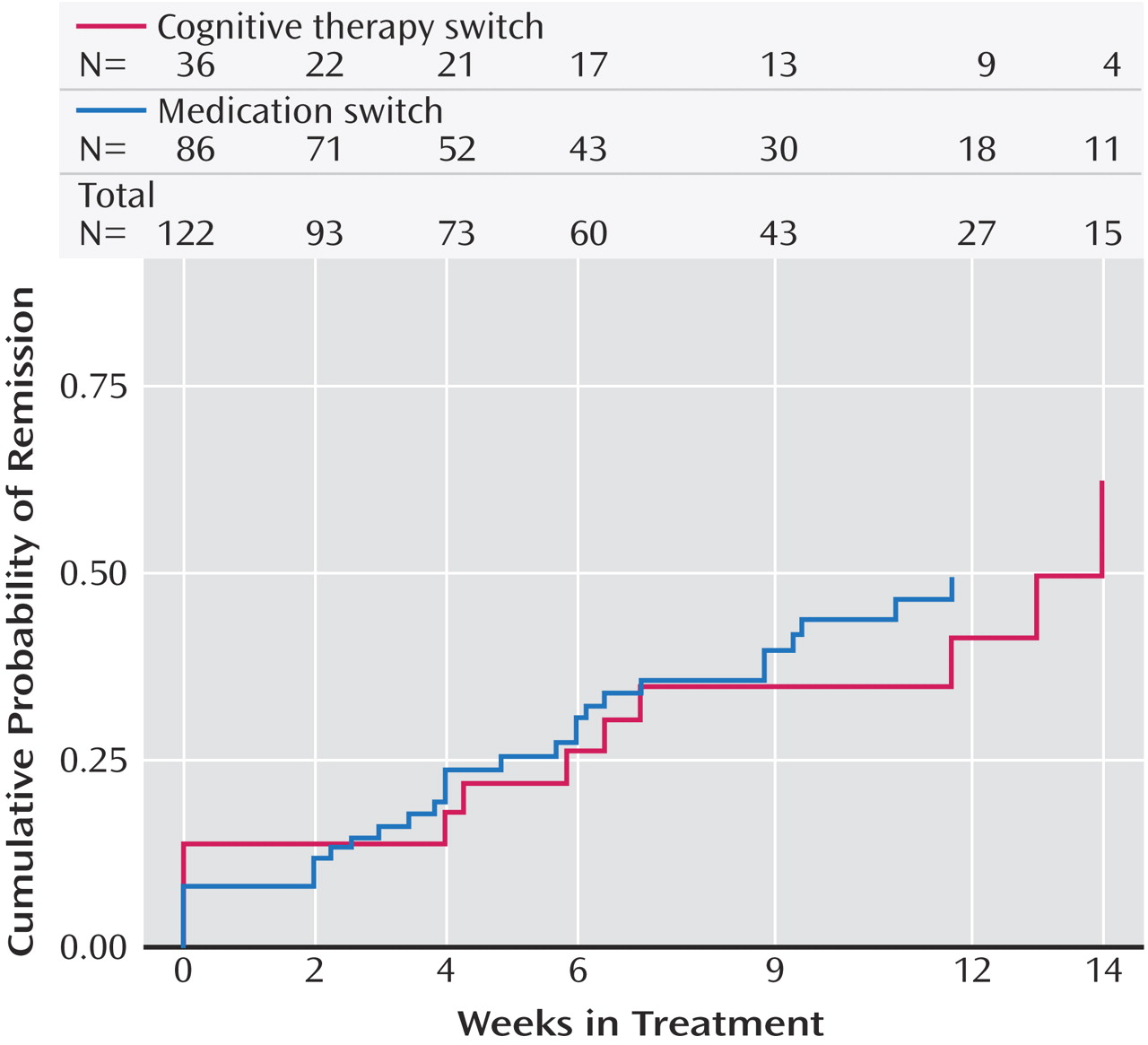
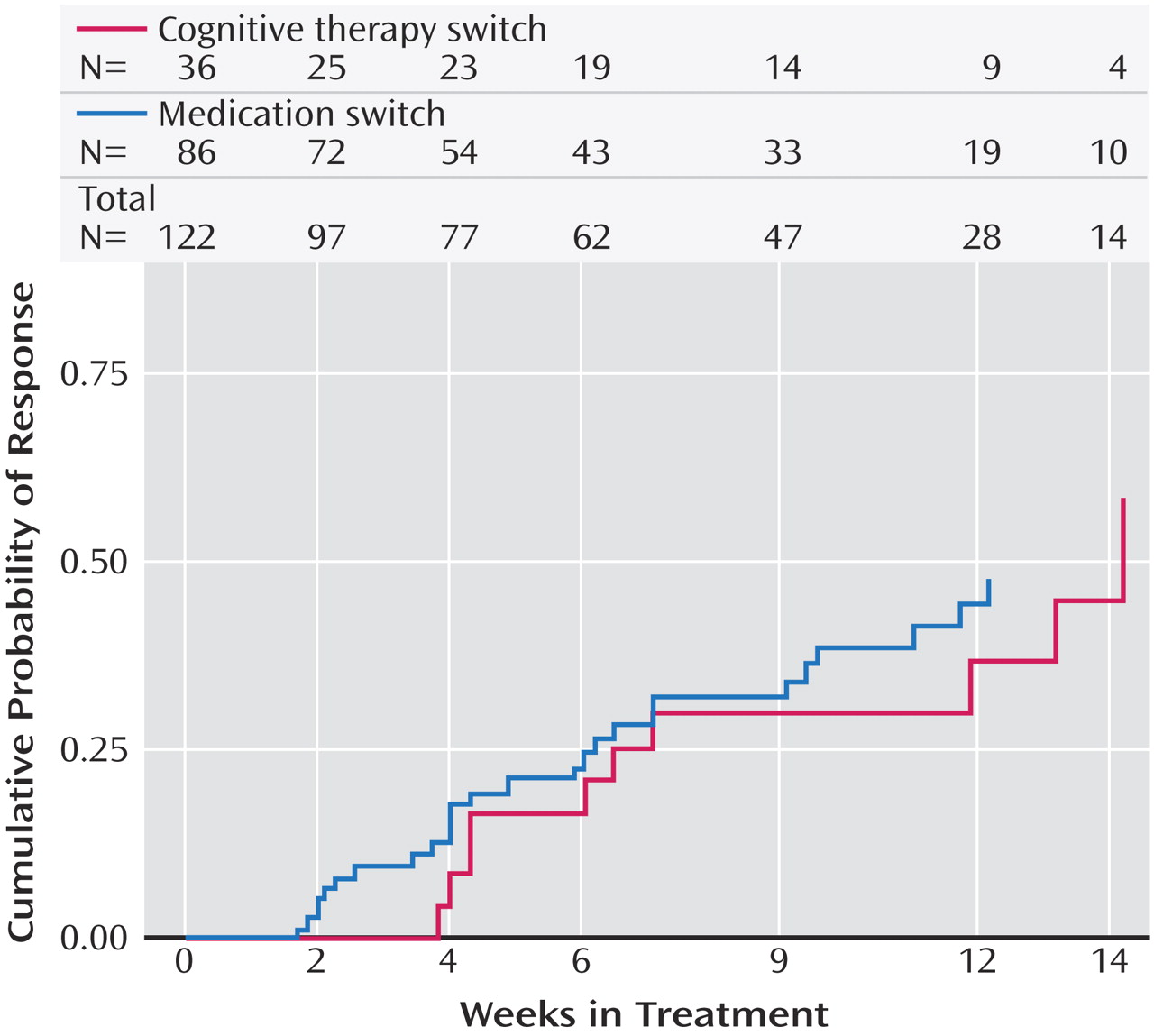
Patients who switched treatments were likewise about as likely to benefit from cognitive therapy as those who were switched to sertraline, sustained-release bupropion, or extended-release venlafaxine. In contrast to the augmentation groups, the difference in speed of remission was not statistically significant. The major difference between switching to cognitive therapy and switching to another medication was that participants who received cognitive therapy alone were spared the side effect burden of a second course of pharmacotherapy. However, given that the percentage of patients who exited the study because of side effects did not differ between the cognitive therapy and medication switch arms, this advantage may be of limited clinical significance. As an aside, we thought it noteworthy that although the U.S. Food and Drug Administration warns of the emergence of suicidal ideation as a hazard following initiation of antidepressant medication, several cases of suicidal ideation occurred as serious adverse events following the initiation of cognitive therapy in our study.
The remission rates we report here for cognitive therapy (either switch or augmentation) are different from those reported previously by Rush et al.
(46) because we used different STAR*D samples. For the cognitive therapy switch remission rates, Rush et al. reported on all participants who received a switch to cognitive therapy, whereas in this article we focused only on those who were both willing to be randomly assigned to cognitive therapy switch or medication switch and were randomly assigned to one of these treatments (see
Figure 1 ; we excluded 24 participants who were willing only to receive cognitive therapy as a switch or augmentation and two who were willing to accept any treatment except medication switch). Likewise, for the cognitive therapy augmentation remission rates, Rush et al. report on all participants who received cognitive therapy augmentation, whereas we focused only on those who were both willing to be randomly assigned to cognitive therapy augmentation or medication augmentation and were randomly assigned to one of these treatments (see
Figure 1 ; we excluded 20 participants who were willing only to receive cognitive therapy as a switch or augmentation).
The outcomes for participants treated with cognitive therapy in this study are generally comparable to those reported by Scott
(13) and Schatzberg et al.
(17), although much less promising than those of Fava et al.
(15) . The latter reported that 12 of 19 patients who had not benefited from antidepressant medication responded to a form of cognitive behavior therapy that emphasized life style management and engagement in healthy, adaptive activities. Although it is plausible that differences in the emphasis of therapy could explain the better outcomes, differences in design (Fava et al. conducted a nonrandomized, single-site case series with a single expert therapist) and patient illness characteristics (Fava et al. excluded patients with medical and psychiatric comorbidities) seem more likely to account for the differences.
The greatest shortcoming of this study is the lack of statistical power to detect moderately sized between-group differences. This low power was a result of lower-than-expected numbers of patients agreeing to randomization strata that included both cognitive therapy and pharmacotherapy. The STAR*D investigators had expected that more patients would be at equipoise on the acceptability of second-step treatments. In fact, one of the most surprising findings of STAR*D overall was that only 1.5% of patients were willing to accept all seven of the second-step options (
Figure 1 ). It was particularly surprising that only 26% of the patients were willing to accept assignment to cognitive therapy as either a switch or an augmentation strategy. These low numbers also limited the ability to control for imbalances in baseline characteristics.
The STAR*D investigators were experienced in designing studies of depression-focused psychotherapies, and the unexpectedly low acceptability of cognitive therapy was frankly at considerable variance with our earlier research experiences (see, for example, references 47–51). For example, even among more severely depressed inpatients, the Pittsburgh group
(52) found that more than three-quarters of eligible patients accepted treatment with an intensive form of cognitive therapy alone instead of pharmacotherapy. Of particular relevance to the current study is the report of Schatzberg et al.
(17), in which chronically depressed patients who did not respond to 12 weeks of therapy with either a form of cognitive therapy or nefazodone were permitted to switch to the other treatment. In that study, 88% of participants who did not respond to nefazodone accepted the switch to psychotherapy, and 95% of those who did not respond to psychotherapy accepted the switch to nefazodone.
Psychosocial interventions likewise are not commonly viewed as “unacceptable” alternatives to pharmacotherapy outside of research studies. In fact, surveys of patients’ treatment preferences have consistently found that counseling and psychotherapy receive higher marks than pharmacotherapy
(53 –
56) . Thus, it may be that some aspects of the design of STAR*D inadvertently biased patients against accepting cognitive therapy as compared with a second course of pharmacotherapy. For example, since the initial treatment in STAR*D was pharmacotherapy, patients who wanted to begin treatment with psychotherapy would very likely have opted not to enroll in the study. A second possible factor is the cost of study treatments. Whereas STAR*D provided the medications and most of the study-related sessions not covered by insurance, it was not possible for the study to reimburse participants for copayment charges for insurance-covered psychotherapy sessions. A third potential bias may have resulted from the need for patients to go to a different site to see a psychotherapist. If so, this is a real-world factor that may limit the utility of psychotherapy as a second-step option, given that referral to a provider at a different site is routine practice.
These were not the only factors that may have dampened patients’ enthusiasm for receiving psychotherapy, however, because cognitive therapy was significantly more acceptable to participants who opted for augmentation than it was to those who opted for a treatment switch. Perhaps the potential fear of breaking the treatment alliance with the study physician biased some patients against accepting a switch to cognitive therapy. Not only did participants in the augmentation groups get to continue citalopram, they also maintained an ongoing therapeutic relationship with their study physician. Finally, because patients who were already participating in psychotherapy could be enrolled in STAR*D, a small number of otherwise eligible participants who wished to continue their current therapy may have declined the option of cognitive therapy with a study therapist. Unfortunately, since we did not systematically record the number and nature of these extraprotocol courses of therapy, it is not possible to determine whether or not this was a common occurrence.
Another limitation of the study is that the fidelity of the therapy was not independently evaluated, and, because not all of the therapists were highly experienced with cognitive therapy, it is possible that some participants did not receive an optimal course of therapy. Both of these limitations reflect the STAR*D investigators’ decision to study the effectiveness of cognitive therapy under real-world conditions. That said, all study therapists were screened to ensure basic competence in cognitive therapy, and they received ongoing supervision—quality controls that probably surpass those generally found in clinical settings. Although it is not certain that expert therapists would have achieved better results with this difficult-to-treat patient population, there is evidence that therapist adherence is associated with better outcomes in cognitive therapy
(57,
58) . In two multicenter controlled studies
(59,
60), site differences in cognitive therapy response suggest that therapists’ experience level may significantly affect outcomes, perhaps particularly for inpatients with more difficult-to-treat depression.
Our study examined only one form of psychotherapy. Other approaches, including interpersonal psychotherapy and the cognitive behavior analysis system of psychotherapy, may have yielded different results. Secondary analyses of the Treatment of Depression Collaborative Research Program suggest that interpersonal psychotherapy performed better than cognitive therapy among the subset of patients with more severe depressive symptoms
(61,
62) .
Finally, less than one-third of the patients remitted with any of the Level 2 treatments in STAR*D, which indicates that there is room for improvement in the treatment of depression. Future analyses will examine the impact of the STAR*D treatments on functioning and quality of life. Given that only about one-fourth of the patients treated with cognitive therapy completed the full 16-session protocol, it would be worthwhile to explore novel methods of facilitating mastery of therapy materials without increasing the number of therapy sessions. It would be useful in future research to determine whether concurrently starting psychotherapy and making changes in pharmacotherapy can result in higher remission rates. It would likewise be worthwhile in future research to study alternative methods of delivering therapy, such as greater use of telephone sessions and supplemental materials (e.g., self-help materials via print, Internet, video, or DVD modules), to determine whether the acceptability of psychotherapy can be improved.