The concept of individuals engaging in behavior with the intent to harm or injure themselves seems unnatural or foreign to the average person. To see a child intentionally and repeatedly strike his or her head and bite his or her arm certainly raises many questions as to what this child experiences. The thought of personally receiving such a blow is intolerable, even painful. Since many of the children who engage in self-injurious behavior have mental retardation, they are unable to convey their perception of the experience. What they do convey, however, is that this behavior both serves a purpose and is perceived differently from what might be expected. A functional and descriptive analysis identifying the precipitants and progression of the behavior can provide some clues to an appropriate treatment
(1), although a beneficial response is not always achieved. We present a case and discussion of a 3-year-old boy with significant self-injurious behavior who responded to treatment with naltrexone.
Case Presentation
Alex (not his real name) was a 3-year-old developmentally delayed son of a Caucasian father and an Asian mother. He was referred for a child neuropsychiatric evaluation for pronounced self-injurious behavior that was unresponsive to behavioral intervention. Before the referral he had completed an extensive evaluation by specialists in the fields of developmental pediatrics, genetics and metabolism, and neurology to elicit the etiology of his developmental delays and cognitive deficits. No significant abnormalities were identified on brain magnetic resonance imaging, chromosomal analysis, or any of the metabolic, urine organic acid, or amino acid screening tests. Alex had a slightly elevated creatine phosphokinase level of 136 and a marginally abnormal acyl carnitine profile, showing elevation of total and free carnitine levels. Results of a full workup for Lesch-Nyhan syndrome was negative. A muscle biopsy was not performed, and thus a mitochondrial disorder was not completely ruled out. However, this was considered a remote possibility.
The onset of Alex’s self-injurious behavior was at approximately 2 years of age. The initial symptoms involved head banging, which was mild and transient but progressively worsened. The behavior subsequently evolved to repetitively slapping his ears, resulting in bleeding fissures in the postauricular regions. He was followed closely during this period by a developmental pediatrician who found no evidence of otitis media or corneal abrasions that might account for the behavior.
As the self-injurious behavior progressed, Alex began biting both his right and left arms. His arms developed calluses and areas of oozing and bleeding where the epidermis was shed. There were no skin lacerations. The frequency of his self-injurious behavior was daily, varying from approximately 1 to 6 hours intermittently, and interfering significantly in family function. Family members reported that they seldom went out as a family and that there was difficulty finding a baby-sitter who would feel comfortable watching Alex while they went out.
The family reported that he appeared in considerable distress during his self-injurious episodes. They also noted that he did not cry or seek comfort during potentially painful childhood experiences, such as scrapes and bruises from falling. During the day Alex attended an early intervention program for children with developmental disabilities. He continued the self-biting behavior with equal severity, whether he was at the early intervention program or at home.
When he was initially seen at our clinic Alex was noted to be very distressed and irritable. He exhibited a moan-like cry as he moved back and forth across the office. Both his verbal and nonverbal communication patterns were significantly restricted, although his parents reported that he did seem to acknowledge their presence at times. Both parents also appeared considerably distressed, describing the difficulties of being unable to consistently protect their child from his own actions.
Alex did not display any specific stereotypies, hand-flapping behavior, or tics. During the evaluation he frequently placed his mouth against his arms, either sucking or pulling at the skin. As a result of this behavior he had multiple ecchymotic lesions and callous formations on both arms. These lesions were most notable on the left arm and covered approximately 70% of the left forearm surface with oozing areas.
Self-injurious behavior, defined as self-directed acts that result in tissue damage
(2), is not uncommon among individuals with developmental delays or mental retardation. The prevalence of self-injurious behavior in this population ranges from 2% to 19% in community samples
(3–
5) and as high as 8%–40% among institutionalized populations
(6–
8). Not only does this behavior adversely affect the patients, but it also wields a significant emotional and physical toll on their caretakers
(9,
10). The majority of individuals have no identifiable medical condition directly attributable to the self-injurious behavior. One rare exception is Lesch-Nyhan syndrome, an X-linked recessive disorder, in which self-injurious behavior is a phenotypic expression of the disorder
(11,
12).
The treatment of self-injurious behavior is a complex and challenging problem. Psychotherapeutic treatment modalities have consisted of social skills training
(13) and behavioral therapies
(2,
14–
44). Moderate or severe self-injurious behavior that proves minimally responsive or nonresponsive to behavioral therapy often warrants a combination of behavioral and psychopharmacologic interventions. A multitude of pharmacologic treatments have been studied in self-injurious behavior
(25–
28), with neuroleptics constituting the most common agents employed
(29,
30). Alternative medications reported to be beneficial in subgroups of individuals with self-injurious behavior include lithium
(31–
33), tricyclic antidepressants
(34), β-adrenergic blockers
(35,
36), trazodone
(37,
38), tryptophan
(37,
39),
l-dopa
(40,
41), tetrabenazine
(41), selective serotonin reuptake inhibitors
(42–
44), buspirone
(45), and opioid antagonists
(46–
68).
The myriad of pharmacologic treatments parallel and, in many cases, are the driving force behind the etiologic theories underlying self-injurious behavior. Such diversity highlights the probable etiologic heterogeneity of self-injurious behavior. The difficulties in treating patients with self-injurious behavior is exemplified in the X-linked condition of Lesch-Nyhan syndrome, in which self-injurious behavior is directly expressed as a genetic phenotype, yet no therapeutic agents consistently alleviate the behavior
(12). When an efficacious response to pharmacologic therapy occurs, it most commonly is the result of medications that influence either the dopaminergic or the endogenous opioid system. These two systems are not functionally independent
(69), and furthermore, it is likely that other neurotransmitter systems, e.g., serotonin and glutamate, also play a role in perpetuating self-injurious behavior
(70).
The medications most commonly employed, including dopamine 2 (D
2) receptor blockers such as haloperidol and thioridazine, have shown to only mildly improve self-injurious behavior symptoms
(71). Newer agents that have a greater affinity for blocking dopamine 1 (D
1) receptors appear to be more effective
(25,
26,
72), although further study is warranted. Opioid antagonists actually have the best overall record and have been shown to be beneficial in reducing self-injurious behavior in 40%–60% of individuals
(73,
74).
During the initial visit no specific decisions were made regarding the treatment course for Alex. His previous medical records were obtained and reviewed. He had received a trial of thioridazine in the past without any notable improvement on low doses (30 mg/day), but he did display decreased self-injurious behavior secondary to sedation as the dose was titrated upward. He also was seen regularly by a pediatric neuropsychologist without any significant improvement after behavioral interventions.
When the family returned for a subsequent visit, the potential benefit of naltrexone hydrochloride was discussed, including the risks, benefits, and theories behind its efficacy. Alex then started treatment with 12.5 mg/day of naltrexone (0.98 mg/kg/day). After 2 weeks of pharmacotherapy the father stated that the self-injurious behavior had worsened to such an extent that a laceration had developed on his left arm requiring intervention from his pediatrician. Alex’s self-injurious behavior and irritability had increased both at school and home. At this juncture the options of either discontinuing or increasing the medication surfaced. The potential ramifications of these options were discussed with the family.
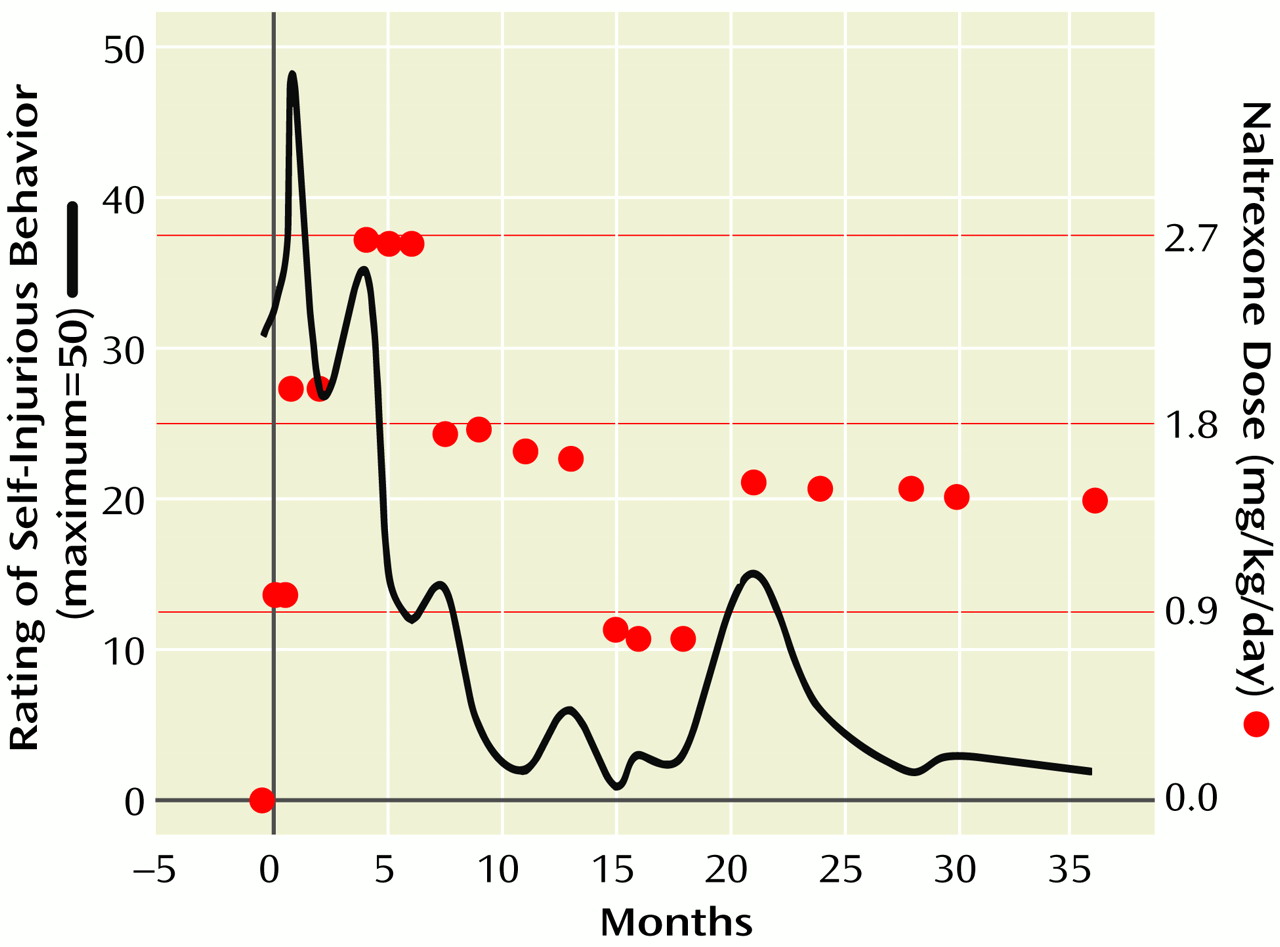
The critical nature of his behavior necessitated making a judgment call about whether to stay the course or abandon naltrexone for an alternative class of drugs, such as neuroleptics. According to the “addiction” theory, the worsening self-injurious behavior could be expected as an “extinction burst,” and increasing the dose might prove beneficial. On the other hand, increasing the dose could potentially further escalate the self-injurious behavior. Alternatively, the worsening behavior could have been a natural progression of his self-injurious behavior, unrelated to the medication. Increasing the dose in the face of worsening self-injurious behavior was not a trivial decision, yet it was mutually decided to closely monitor Alex’s self-injurious behavior while titrating the dose upward. The naltrexone dose was increased to 25 mg/day (1.97 mg/kg/day), and over the course of the next month the self-injurious behavior gradually improved (
Figure 1. ).
One theory supporting the use of opioid antagonists postulates that self-injurious behavior becomes linked to endogenous endorphin release in an operant conditioning, or “addiction” paradigm
(47,
52). In this addiction hypothesis, the self-injurious behavior is thought to be initially unrelated to the mechanism of endorphin release, but it becomes cumulatively reinforced through operant conditioning. The triggering behavior may include excessive self-stimulatory behavior
(75), initiated as a means to either avoid or obtain attention
(76), as a form of communication, or to compensate for a dopamine regulatory defect
(27).
For example, the patient initiated his self-injurious behavior with head banging, which is not uncommon among children. Children with otitis media or other painful experiences may engage in head banging to displace their discomfort. When the underlying problem is treated, the head banging typically subsides. However, children with developmental delays may not be able to communicate their discomfort, and thus head banging could serve as a distracter. Over time, however, the release of endogenous endorphins not only masks the discomfort but also may generalize to mask other forms of discomfort or even be a source of comfort or euphoria.
Studies increasingly demonstrate support for the addiction model in at least a subgroup of individuals with self-injurious behavior. Animal models and human studies both have demonstrated that endogenous endorphins are released in the presence of painful or stressful events
(77–
79). Tolerance and dependence have been shown to develop at the release of β-endorphins
(80). Furthermore, plasma β-endorphin levels have been shown to be elevated in patients immediately after an episode of self-injurious behavior compared with baseline levels
(73,
81).
Both β-endorphin and ACTH are cleaved from the larger proopiomelanocortin peptide, and thus levels are typically highly correlated
(82–
84). Since ACTH is a hormone released in response to stress, the increased self-injurious behavior could merely be a reflection of the stress associated with the injury itself
(82,
85). However, although healthy subjects undergoing a stressful event show a high correlation between β-endorphin and ACTH levels, patients engaged in self-injurious behavior display an uncoupling of this correlation, i.e., a rise in plasma endorphin levels without an associated rise in ACTH
(81). A similar uncoupling of β-endorphin and ACTH has been demonstrated both in heroin addicts as a response to stressful stimuli
(86,
87) and in alcoholics
(88). Proopiomelanocortin-derived peptides have been shown to display some variability in their release
(83), although the mechanisms are not well understood.
Even in the presence of β-endorphin release in the context of a stress reaction, a major issue is whether peripheral β-endorphin release has related or resultant central nervous system (CNS) effects. Little of the peripherally secreted β-endorphins actually cross the blood-brain barrier and influence CNS β-endorphin concentrations
(89–
91). However, it is possible that the post-self-injurious behavior increase in β-endorphins also has a CNS component. Inder et al.
(88) used the ACTH incremental response to naloxone as a marker for central opioid tone and found a significant correlation with basal β-endorphin immunoactivity. They postulated that similar regulatory mechanisms take place on both sides of the blood-brain barrier and opioid-active β-endorphin predominantly affects the CNS and nonopioid-active β-lipotropin, a precursor of β-endorphin, secretes from the pituitary into the plasma. Thus, although plasma β-endorphin levels are elevated after self-injurious behavior, many unanswered questions remain regarding the relationship between peripheral and central opioid levels.
Early treatment models utilizing an aversive remote shocking device found that some individuals would purposefully engage in self-destructive behavior to receive an electric shock
(73). After receiving the aversive stimuli, they would not engage in any further self-injurious behavior until they needed another “fix.” This notion of addiction, or the need for a fix, was described by Thompson et al.
(70), who found similar patterns between self-injurious behavior “administration” and cocaine and morphine self-administration. Given these similarities, it is not surprising that both dopamine and β-endorphins are implicated in substance abuse disorders
(92–
95).
If the addiction model is valid, a natural consequence of blocking the self-administered endorphin would be what B.F. Skinner described as an “extinction burst”
(96), namely, an increase in self-injurious behavior in an attempt to override the β-endorphin block
(48,
97). Both animal models and human studies demonstrate drug-seeking behavior and withdrawal symptoms after the discontinuation of long-term drug ingestion
(77). Although several of the treatment studies with opioid antagonists report an extinction burst
(47,
50,
62,
97), it does not occur in the majority of cases. This lack of an extinction burst in a considerable proportion of the cases reported has been an argument against the addiction model of self-injurious behavior
(98).
The patient, however, demonstrated what could be considered a fairly classic extinction burst. He exhibited worsening behavior coupled with increasing distress, as though he were experiencing withdrawal. The decision to increase the dose was based on the supposition that the patient required an uncoupling of self-injurious behavior with the comforting effects of β-endorphin self-administration. Certainly the risk of worsening self-injurious behavior could have required cessation of the naltrexone, as was required in the case reported by Benjamin et al.
(97). It should be also noted that the patient’s worsening behavior and subsequent improvement could have been a developmental progression that may have taken place without pharmacotherapy. Environmental factors may also have contributed, although the family did not know of any specific environmental stressors. Furthermore, before pharmacotherapy the patient clearly had a self-injurious behavior trajectory that was gradually worsening.
Approximately 3 months after the successful medication increase, Alex exhibited a recurrence of worsening self-injurious behavior. His naltrexone dose was titrated up to 37.5 mg/day (2.68 mg/kg/day), and his self-injurious behavior gradually remitted. He remained at this dose for 4 months while revealing a significant decline in self-injurious behavior. Over time his ecchymotic and bleeding areas gradually healed. Of equal importance was that his irritable overlay dampened considerably, and his social communication showed mild improvement. On several occasions he also reacted adversely to blood sampling.
He continued to take naltrexone over the subsequent 3 years, albeit with some dose changes (
Figure 1. ). He occasionally developed episodes of breakthrough ear slapping or head banging in the absence of an upper respiratory tract infection, otitis media, or other medical problem. In each instance a dose adjustment resulted in a cessation of the behavior. The highest dose he received was 37.5 mg. Liver enzymes, which were checked monthly for the first 6 months and then quarterly, remained stable.
A second theory for the role of endogenous opioids in reducing self-injurious behavior is the “pain” or “analgesia” model
(52). This theory postulates that there are high levels of circulating β-endorphins that override or significantly decrease the experience of pain. The self-injurious behavior is thus considered another form of self-stimulatory behavior, and the pain associated with the self-injurious behavior is masked by means of the endogenous opiate system. Higher levels of β-endorphins were found in both patients who engaged in self-injurious behavior
(99,
100) and those who demonstrated self-stimulatory behavior
(99). Higher rates of self-injurious behavior corresponded to higher levels of β-endorphins
(100). Animal studies have demonstrated that by iatrogenically increasing the pain threshold by means of morphine or sufentanil administration to rats caused them to exhibit self-injurious behaviors
(101,
102). Furthermore, opiate receptor blockers reverse congenital insensitivity to pain
(103). Thus, according to this theory, naltrexone serves to reduce the elevated pain threshold, so the self-injurious behavior diminishes as the individual begins to experience pain associated with the behavior. Opioid antagonists have been shown to both reverse pain insensitivity and lower the pain threshold
(104,
105). Since the high levels of circulating β-endorphins merely mask the pain associated with self-injurious behavior, an extinction burst would not be an expected outcome
(48).
The patient initially lacked a distressed response to blood sampling and to injuries that would be considered painful for children of his age. High circulating levels of endorphins could mask these painful events. The majority of individuals with self-injurious behavior do not exhibit even the slightest signs of experiencing pain during either their self-injurious behavior episodes or after apparent painful experiences, e.g., blood sampling
(73). In several case studies, including the one reported here, distress to painful stimuli (i.e., blood sampling) first surfaced with the use of an opioid antagonist. Thus, it is conceivable that their efficacy is related to lowering the pain threshold. Alternatively, the patient’s high frequency of self-injurious behavior could have accounted for high endorphin levels that masked his pain, especially since he had self-injurious behavior without significant self-stimulatory behavior. Finally, a combination of the addiction and pain hypotheses could play a role.
As an alternative to direct receptor blockade, opioid antagonists may bring about a reduction in self-injurious behavior through an interaction with dopaminergic pathways
(9). Naltrexone was shown to have a therapeutic window in blocking pemoline-induced self-biting behavior in prepubertal rats
(9). In light of the presence of a co-localization of dopamine and opioid neuron receptors
(106), the efficacy of naltrexone in self-injurious behavior may involve both opioid and dopaminergic mechanisms.
A complex interplay exists between the endogenous opioid and dopaminergic systems that is not fully elucidated. Stimulation of opioid receptors in the ventral tegmental area increases dopamine levels in the nucleus accumbens
(107–
109). Increased dopamine levels in the nucleus accumbens have been shown to be related to the euphoric feelings associated with morphine administration
(110). Furthermore, Breese et al.
(111) demonstrated that the prenatal ablation of dopaminergic neurons in the ventral tegmental area resulted in the later development of self-injurious behavior in rats. This self-injurious behavior was effectively blocked by means of a D
1, not a D
2, antagonist
(112). Additionally, a recent study of dopamine-opioid interactions in the hypothalamus reported that D
2 dopaminergic pathways have a predominantly inhibitory effect on the endogenous opioid system
(113).
It thus appears that the mesolimbic dopaminergic system plays a role in the symptoms of at least a subgroup of those with self-injurious behavior. Yet interpreting the data describing the β-endorphin–dopamine interaction is likely to be oversimplistic. Not only are there multiple receptors for both the β-endorphin and dopamine systems, but it is likely that other neurotransmitters also modulate the interaction of these systems. Nevertheless, if D
2 dopaminergic pathways have a generalized pattern of inhibiting the opioid systems, ablation of the ventral tegmental area could cause an up-regulation of the opioid system in the nucleus accumbens. In this scenario, self-injurious behavior may reflect an insensitivity to pain related to chronic hypoalgesia
(52,
99). Alternatively, with an intact ventral tegmental area, the β-endorphin pathways interact with dopamine as would exogenous opioids such as morphine, thus reinforcing the behavior that prompts the endorphin release.
Each of these models has reasonable research support, and each sheds light on the probable multiple factors involved in self-injurious behavior. Yet treatment decisions, such as how long to continue opioid antagonists, remain empiric and based on individual cases.
After 8 months of treatment Alex’s naltrexone dose was gradually tapered from 37.5 mg/day (
Figure 1. ). Alex experienced a slight worsening of his symptoms but then did well taking 25.0 mg/day. After a year another taper was initiated; however, his behavior worsened, requiring an increase in naltrexone to 25.0 mg/day. When Alex neared the point of discontinuation he began head banging, and his parents became worried about a return of his self-injurious behavior. He has continued taking naltrexone for approximately 3 years without resurgence of his self-injurious behavior. A subsequent taper was planned; however, the father, an engineer, was transferred to Asia. It was decided that the taper would best be done by his new physician after Alex’s adjustment to the move.
Naltrexone is considered a pure opioid antagonist because it blocks the effects of opioids by competitively binding at opioid receptors. At low doses, naltrexone binds to the mu receptors within the CNS. At higher doses, naltrexone binds to the kappa and delta opioid receptors, which may modify the mu opioid receptor, resulting in a loss or decrease in effect. Evidence exists for a therapeutic window with naltrexone dosing
(9,
48). The usual dose of naltrexone in self-injurious behavior is 0.5–2.0 mg/kg/day in children and adolescents. Herman et al.
(49) described a U-shaped dose-dependent curve with a diminishing effect once the dose exceeded 1.5 mg/kg/day. The patient did not appear to exhibit a U-shaped dose-response curve, although the higher metabolic rate in children precludes mg/kg/day comparisons with adult studies. The patient did require dose adjustments, which is not uncommon in individuals with self-injurious behavior
(73), and his highest dose was 37.5 mg/day (2.68 mg/kg/day). Finally, it is conceivable that the patient’s initial worsening was a low-dose effect (i.e., a low-dose interaction of the mu, delta, and kappa receptors), rather than an actual extinction burst. Sandman et al.
(81) noted that a number of patients had a worsening of their self-injurious behavior at doses of 0.5 mg/kg/day but improved when the dose was increased to 2.0 mg/kg/day.
The majority of studies of opioid blockers evaluated the change in self-injurious behavior over weeks rather than months or years. Two studies have shown that a subgroup of individuals continue to improve with long-term treatment
(73,
74), and these gains may continue once naltrexone has been discontinued
(114). After the initial worsening of self-injurious behavior, the patient showed continued improvement over the next year. Although hepatotoxicity is a potential adverse effect of naltrexone therapy, the patient’s liver function test results remained within normal limits during the 3 years of treatment, as has been noted in other studies
(58,
67,
68).
Conclusions
Both animal and human studies provide clues to the neurodevelopmental and neurochemical underpinnings of self-injurious behavior. Yet the many ways that a person engages in self-injurious behavior is exceeded perhaps only by the number of available treatments, which are as numerous as the theories supporting each treatment. It is clear that self-injurious behavior is complex and multifaceted, and it is not surprising that successful treatment is often elusive. Yet studies demonstrating that 40%–60% of individuals with self-injurious behavior improve with treatment with opioid antagonists are reassuring to clinicians and families.
To date there have been a total of 40 studies evaluating the efficacy of opioid antagonists in the treatment of self-injurious behavior
(46,
115). Only six of these studies contained over five individuals
(58,
60,
64,
66,
68,
115), and five of these were double-blind studies
(60,
64,
66,
68,
115). These double-blind studies had mixed results; three studies showed considerable improvement
(60,
66,
68), and the remaining two exhibited no improvement
(64,
115). The largest double-blind study (25 individuals) failed to show a significant decline in self-injurious behavior with naltrexone
(115). However, the results of a larger retrospective study, which drew from the entire state school population in Texas, supported the clinical efficacy of naltrexone. In this study, Casner et al.
(74) found that over 50% of the 56 individuals treated with naltrexone were considered to have a beneficial response and remained with treatment for the long term.
In attempts to tease apart the heterogeneity of treatment response, researchers have reported variations in improvement depending on the specific type of self-injurious behavior. There is not only intersubject variability in the response to specific forms of self-injurious behavior; intrasubject variability also exists. For example, naltrexone was shown to have better intrasubject efficacy in reducing blows to the head
(116,
117) and face
(116) but had little effect on self-biting
(116) and head banging
(117). Thompson et al.
(68) reported a decrease in blows to the head with little effect on eye, nose, or throat poking. This differential response of naltrexone to specific forms of self-injurious behavior within the same individual is perplexing.
Several theories have been described to explain the selective response of naltrexone to different forms of self-injurious behavior. Since a selective treatment response to specific forms of self-injurious behavior has also been demonstrated with D
1 blockers
(118), Thompson et al.
(68) have speculated that specific forms of self-injurious behavior may be modulated by different neurochemical pathways, even within the same individual. This hypothesis is supported by specific body sites being better sources for analgesia or opioid release, such as acupuncture
(68,
119). To our knowledge, there are no studies to date, however, showing treatment response variability to different medications within the same individual. Environmental factors could be involved in both initiating and propagating self-injurious behavior (e.g., increased attention from parents or staff)
(76). Medication effects would not necessarily influence these behaviors. Finally, Herman et al.
(116) pointed out that changes in low-frequency behaviors may have more difficulty reaching statistical significance.
The patient initiated his self-injurious behavior with head banging, but over time this changed to ear slapping and then to biting his arm. Under the addiction model, the change in his self-injurious target behavior could be a result of “dose effect,” with different target regions providing different endogenous opioid responses. The change in self-injurious target behavior may also reflect a change in self-stimulatory behavior, which is not uncommon among children with developmental conditions. Under the pain hypothesis, the perception of pain in this second scenario would be masked by elevated endorphin levels. The role of change in target behaviors versus medication response has not been addressed in the literature, to our knowledge.
One noteworthy point in the clinical case described here is the young age of the child and the short duration of self-injurious behavior (9 months). Willemsen-Swinkels et al.
(115) noted that the failure of naltrexone treatment in their large study could be a result of the long duration of self-injurious behavior and heterogeneity within their study population. Animal models have demonstrated a developmental window in naltrexone efficacy, with a delay in treatment resulting in an absence of efficacy
(120,
121). However, in reviewing 26 of the studies in which the duration of self-injurious behavior was recorded, there was no significant difference in treatment efficacy when comparing those with self-injurious behavior of more or less than 5 years (p=1.00, Fisher’s exact test). Nor was there any age effect on those who demonstrated an extinction burst (p=0.54, Fisher’s exact test). Of the four case reports that described a complete cessation of self-injurious behavior, only one exhibited a duration of self-injurious behavior of less than 5 years. Thus, the short duration of self-injurious behavior and the presence of an extinction burst, as described in our patient, may or may not have accounted for the complete cessation of his self-injurious behavior with naltrexone treatment. Future studies are needed to delineate the relationship between age at onset, duration of self-injurious behavior, and the effectiveness of various treatment modalities.
In summary, we presented a case report of a 3-year-old boy with mental retardation and self-injurious behavior. The self-injurious behavior was not responsive to behavioral interventions. The initial onset of self-stimulatory behavior followed by self-injurious behavior is consistent with the sequence of events predicted by the addiction theory. The self-injurious behavior showed clear worsening over a 2-week period after the addition of naltrexone (i.e., a probable extinction burst) and completely disappeared over months. These gains have been maintained over a 3-year follow-up period. This case highlights the utility of the existing research on self-injurious behavior in clinical decisions. Given the extreme emotional and physical burden that self-injurious behavior inflicts on patients and families, adding to the existing research on the environmental and biological underpinnings of self-injurious behavior is a worthwhile goal.