Depression is common in Alzheimer’s disease and has serious consequences for patients and their caregivers
(1–
4). However, the efficacy of antidepressant therapy for these patients is uncertain
(5–
7). Further study is necessary to confirm that antidepressants, particularly agents with more favorable side effect profiles, are superior to placebo in the treatment of depression complicating Alzheimer’s disease. We present results from a clinical trial whose aim is to investigate the benefits of antidepressant therapy for patients with Alzheimer’s disease who also suffer from major depression.
Method
Participants were 22 outpatients with probable Alzheimer’s disease according to National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria
(8) who also met DSM-IV criteria for major depressive episode. The patients were recruited from the outpatient clinics of the Johns Hopkins Neuropsychiatry Service. To address potential overlap between the symptoms of Alzheimer’s disease and major depression, if DSM-IV criterion 2 were to count toward the diagnosis of major depressive episode it had to be clearly because of loss of pleasure (anhedonia) and not entirely because of loss of interest. Also, if DSM-IV criterion 8 were to count toward the diagnosis, it had to be because of indecisiveness and not entirely because of difficulty concentrating. All participants and/or their legal representative provided written informed consent for participation under the oversight of the Johns Hopkins Joint Committee on Clinical Investigation.
Inclusion criteria were a score of 10 or higher on the Mini-Mental State
(9), residence in a community setting, and a caregiver willing to accompany the participant to all visits. Patients also had to be in sufficiently good health that they could be treated safely with sertraline. Potential participants were excluded if they had a current unstable medical condition (e.g., symptomatic uncontrolled diabetes), if they had a medical condition that would preclude use of sertraline (e.g., severe headaches or liver disease), or if they had a condition that affected their ability to participate (e.g., cancer requiring frequent medical visits for chemotherapy). Patients who required hospitalization for depression or for suicidality were also excluded. To promote the generalizability of study findings, every effort was made to include the kind of patient who would be offered sertraline to treat their depression in usual clinical practice.
Patients and caregivers received illness education, encouragement, and emotional support at all study visits. Participants were first given placebo. They continued in the study only if they met eligibility criteria 1 week later, when they were randomly assigned to receive either sertraline or placebo. Clinical follow-up and outcome assessment took place every 3 weeks thereafter. Between clinic follow-up visits, brief telephone contact was made weekly for support and safety monitoring.
Twelve of the 22 patients were randomly assigned to receive sertraline and 10 to receive placebo. Sixteen patients completed all 13 weeks of the study. Two dropped out after 3 weeks of double-blind treatment, one after 6 weeks, and three after 9 weeks. The dropout rates in the two groups were comparable according to a chi-square analysis (p>0.10).
Participants were provided identical-appearing sertraline or placebo capsules under the close supervision of their caregivers. Patients, caregivers, and investigators (except the investigational pharmacist) were all blind as to medication or placebo. All patients were given a starting dose of 25 mg/day of either sertraline or placebo; this was increased to 50 mg/day 1 week later. Doses were increased steadily to a total maximum of 150 mg/day or the highest tolerated dose. The maximum dose of 150 mg/day of sertraline was based on the previous experience of the investigative team that patients with Alzheimer’s disease rarely tolerate sertraline in higher doses without substantial side effects. The upward titration was completed by the 6th week of the study. After week 6, only downward titration for substantial side effects was allowed. The mean peak treatment dose for patients in both groups was 81 mg/day (range=25–125 mg/day).
The principal outcome measure was response to treatment, rated by two study psychiatrists (P.V.R. or M.S.), who reviewed patients’ scores on baseline and follow-up depression rating scales after the patients completed the study. These psychiatrists were blind as to medication or placebo and did not personally manage any patient in the study. They rated each patient on a 3-point global scale as nonresponder, partial responder, or full responder. No algorithm was involved. Rather, the psychiatrists were asked to use their best clinical judgment in making the rating. If there was disagreement between the two raters, they met to provide a mutually agreeable rating.
Another principal outcome measure was the Cornell Scale for Depression in Dementia
(10), given at baseline and at weeks 3, 6, 9, and 12 after treatment initiation. Secondary outcome measures, rated at the same time points, were the Hamilton Depression Rating Scale, the activities of daily living subscale of the Psychogeriatric Dependency Rating Scales
(11), and the Mini-Mental State
(9) to assess global cognitive impairment.
An intent-to-treat analysis was conducted, with the last observation carried forward. Fisher’s exact test was used to compare response rates. Change-from-baseline comparisons on all outcome measures are reported. Changes from baseline differences between the placebo and sertraline groups were compared by using repeated measures analysis of variance (ANOVA) with the baseline score as a covariate.
Results
The 22 study participants had a mean age of 77 years (SD=8.4); 13 were women, and five were nonwhite. There were no differences between the placebo and sertraline groups at baseline on any variable (p>0.20 in all comparisons).
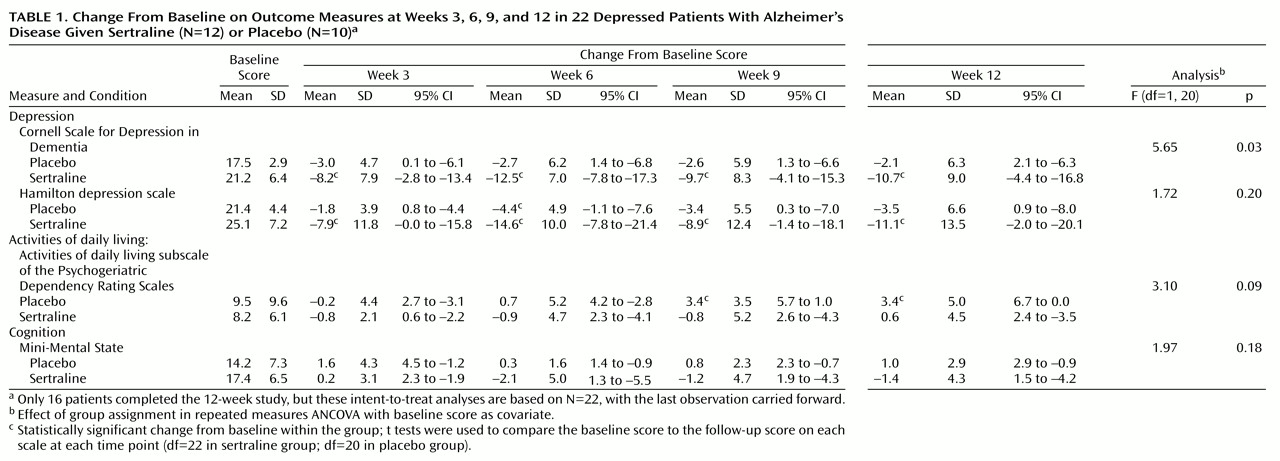
Table 1 shows the mean scores on the measures applied for participants in both groups at baseline.
Study psychiatrists rated three of the 12 patients given sertraline as full responders and an additional six as partial responders, compared with only one full responder and one partial responder among the 10 patients given placebo. This difference in response rates between the two groups was statistically significant (p<0.05, Fisher’s exact test).
Table 1 also summarizes changes from baseline findings at each follow-up point, showing statistically significant within-group changes from baseline and significant between-group differences according to repeated measures ANOVA, with the baseline score as a covariate.
The placebo-treated group had mean reductions of 2.1–3.0 in scores on the Cornell Scale for Depression in Dementia, compared with mean reductions of 8.2–12.5 in the sertraline-treated group. The differences between the groups given placebo or sertraline were significant (
Table 1). Similar significant reductions from baseline were noted in Hamilton depression scale scores in the sertraline group but not the placebo group. However, differences between the groups were not significant for Hamilton depression scale scores according to ANOVA.
On the activities of daily living subscale of the Psychogeriatric Dependency Rating Scales, sertraline-treated patients had no significant change from baseline at any follow-up point. However, the patients given placebo had a significant decline in activities of daily living at 9 and 12 weeks. With regard to the Mini-Mental State, the two groups were comparable at all follow-up points.
Five patients in the sertraline group and two in the placebo group reported nervous system side effects such as tremor and restlessness. Five patients in the placebo group and three in the sertraline group reported gastrointestinal side effects. These side effects were generally mild and well tolerated. Differences in side effects between the two groups were not statistically significant. One patient receiving sertraline developed delirium, and one patient receiving placebo died.
Discussion
In this randomized clinical trial patients with Alzheimer’s disease and major depression were treated with sertraline or placebo. There were significantly greater improvements in mood among patients who received sertraline than among those who received placebo, and response rates were significantly higher for sertraline patients. The bulk of antidepressant response was evident after 3 weeks of treatment. On secondary outcome variables, patients treated with sertraline appeared to be protected against the declines in activities of daily living that occurred in those who were given placebo.
Study findings are consistent with those of two previous studies
(6,
7) but different from those of Reifler et al.
(5), who reported that imipramine was not superior to placebo in the treatment of major depression in Alzheimer’s disease. To our knowledge, ours is the first report on the efficacy and safety of a selective serotonin reuptake inhibitor in patients with Alzheimer’s disease who also have major depression. We did not replicate the findings of Taragano et al. (12) or Rao and Lyketsos (13), who found improvements in Mini-Mental State scores as depression improved in Alzheimer’s disease.
In summary, major depression, a frequent and serious complication of Alzheimer’s disease, responds to antidepressant therapy with sertraline. Reduction in depression with sertraline involves limited risk to patients.