Cognitive deficits persist despite pharmacotherapy and severely limit the recovery of patients with schizophrenia
(1). With coaching
(2–
7) or repetitive practice
(8,
9), patients with schizophrenia are able to make significant task-specific performance gains, and there is some evidence
(10) of generalization to new tasks. Individually specific changes in regional brain blood flow were found in two patients performing a verbal fluency task after intensive neurocognitive training; these changes were thought to be related to different task strategies adopted by each patient after training
(11).
The present study evaluated the effect of repetitive, progressively more difficult exercises on verbal working memory deficits of clinically stable, chronically ill outpatients with schizophrenia. In previous studies
(12,
13), we identified a subgroup of patients with schizophrenia who performed normally on tests of auditory attention and auditory nonverbal memory but had deficits in auditory verbal memory. Similar patients were selected for this pilot study, on the assumption that their attentional competence would make them more likely to benefit. In a previous functional magnetic resonance imaging (MRI) study
(14), we demonstrated that 1) healthy comparison subjects showed statistically significant activation of the left inferior frontal cortex while doing a verbal memory task; and 2) the subgroup of patients with schizophrenia who performed normally on tests of auditory attention and auditory nonverbal memory but had deficits in auditory verbal memory showed significantly less activation of the left inferior frontal cortex during the task. In the present study we used functional MRI to test the hypothesis that training-related improvements in verbal memory are associated with increased activation of the left inferior frontal cortex.
Method
Eight patients (four men, four women) completed the study. All met the following criteria: DSM-IV diagnosis of schizophrenia or schizoaffective disorder, right-handed, no history of alcohol or substance abuse in the past 6 months, no history of neurological disorder or trauma, normal performance on a test of auditory attention
(12), and performance on an auditory verbal memory serial position task that was one or more standard deviations below the means of healthy comparison subjects in our previous studies
(12,
13). Of 19 patients who volunteered for the study, seven were excluded for poor performance on the test of attention and two for normal performance on the test of verbal memory. Two subjects began the protocol but were unable to fulfill the daily training requirement. The mean age of the eight subjects who completed the protocol was 46 years (range=35–54), their mean total Positive and Negative Syndrome Scale score was 48 (range=30–71), and their parents had a mean of 13 years of schooling (range=8–20). The patients had been hospitalized more than five times on average. All were taking medication. Subjects gave written informed consent to participate in the study.
Training consisted of two different auditory verbal serial position memory tasks done in four or five 30–40-minute training sessions per week for 10 weeks. In both tasks, a short list of two to six words is presented, and after a 1–14-second delay one of the words is repeated. Subjects indicate where the repeated word was in the original sequence of words (i.e., first, second, third, etc.). In one task, different words are used on each trial. In the other, the same words are used on all trials, with the order of presentation varied from trial to trial. Individual test trials are randomly generated each day. All subjects began training with two-word lists on both tasks. Task difficulty was increased for each subject individually: when a subject was able to obtain a score of 90% or higher for 3 consecutive days at a given list length, the list length was increased by one word. Patients received performance-based monetary rewards daily but no coaching. By the end of 10 weeks, two patients had attained a list length of six words on one or both tasks, one patient had attained list lengths of five words, and five patients had attained list lengths of four words.
One patient who showed particularly robust performance improvements after the 10-week training period was given 5 additional weeks of training, beginning 6 weeks after the initial 10-week training.
All patients were given four tests of memory before and after training: 1) a five-word version of the training serial position memory task with different words on each trial; 2) a four-word version of the training serial position memory task with the same words on each trial; 3) a four-word visual serial position memory task with different words on each trial; and 4) a three-tone auditory serial position memory task with different tones on each trial
(12). Seven of the patients received this four-test behavioral outcome battery on two occasions approximately 1 week apart before they began training; these scores were averaged to provide baseline values. The eighth subject received the battery only once before training. All eight patients received the battery during the end of the 9th or the beginning of the 10th week of training.
We assessed regional brain activation during the auditory four-word serial position memory task with functional MRI before and after training, using procedures described previously
(14).
Because hypotheses of training-related changes in memory and the relationship between changes in memory and changes in the inferior frontal cortex activation were unidirectional, tests of significance were one-tailed.
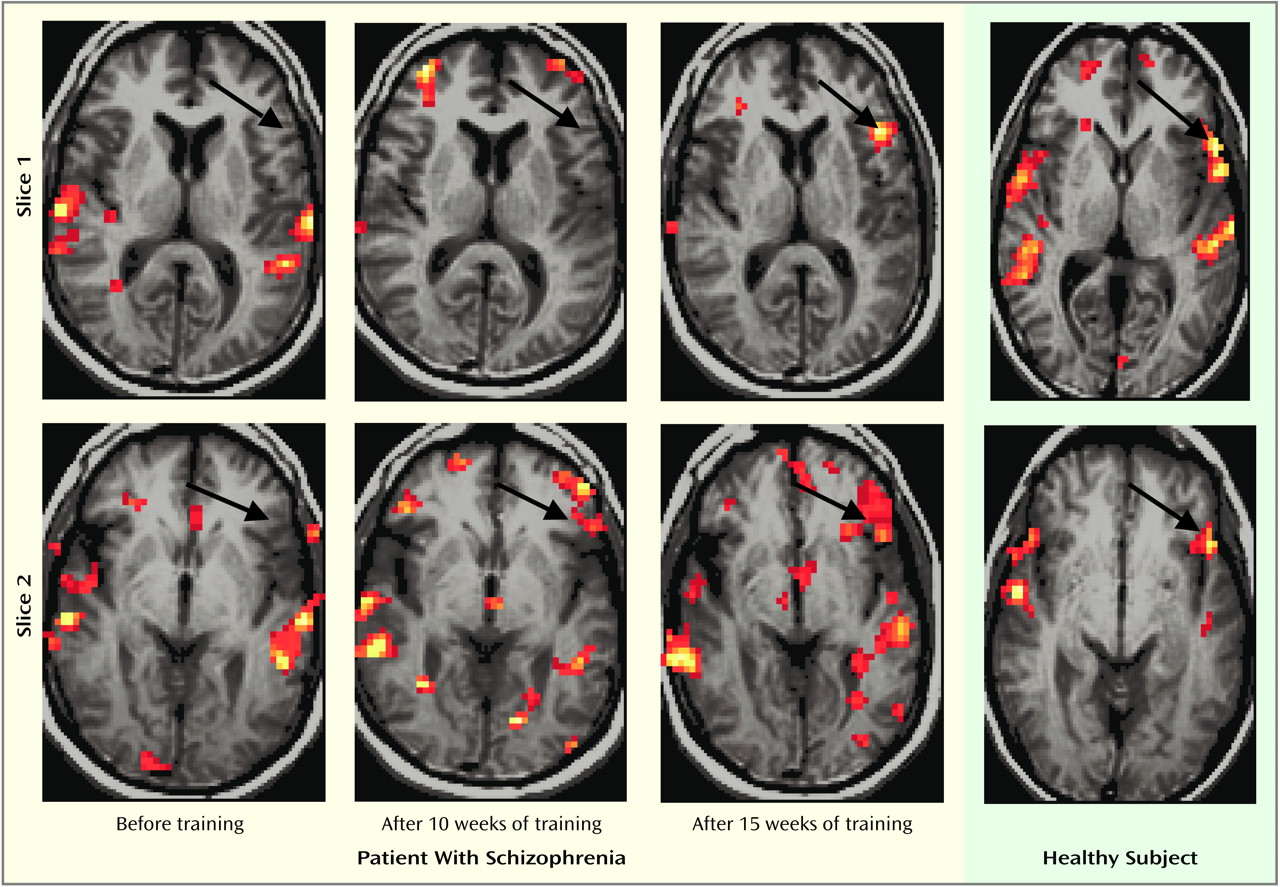
Figure 1 presents functional MRI activation maps obtained before and after training from the patient with the greatest improvement as well as maps from a typical healthy subject from our previous study
(14) for comparison. The statistical significance of the reduced (or absent) task-related activation in the inferior frontal cortex of patients with schizophrenia and its presence in healthy subjects has been established
(14). The maps in
Figure 1 are presented to demonstrate the marked increase in task-related activation that is possible in a patient in a region specifically shown to be underactive in repeated previous studies of schizophrenia (e.g., references
14 and
15). T value thresholds are set at levels that allow areas of greatest signal change to be identified and signal change to be visible in areas expected on the basis of previous knowledge. The actual values are markers of relative magnitude of signal change rather than measures of statistical significance.
Results
Verbal memory test performance gains with training approached significance for the entire group of patients (paired t=1.54, df=7, p<0.09), but performance on the nonverbal tone test was essentially unchanged (68% versus 69% correct). Performance gains on the verbal tests varied among individual patients; three had moderate to large increases (16%, 28%, and 38%), and five had small changes, ranging from a decrease of 10% to an increase of 9%. Two of the patients who showed improvement in verbal memory had scores that were higher than the mean values for the healthy subjects in our previous work
(13) after training, and the third remained more than two standard deviations below the means of healthy subjects despite training-related improvement.
Inspection of functional MRI activation maps before and after training indicated that the patients who showed performance gains also showed increased task-related activation in the left inferior frontal cortex as predicted. The Pearson correlation between behavioral improvement and increased activation of the left inferior frontal cortex was 0.66 (df=6, p<0.04). The scatterplot revealed a highly consistent association between performance improvement and activation increase in all but one patient. This patient was also distinguished by marked ventricular enlargement, with total ventricular volume more than 16 standard deviations above that of healthy subjects in our morphometric laboratory and seven standard deviations above other patients in this study. When this patient was removed from the analysis, the correlation increased to 0.90 (df=5, p<0.003).
The patient who was followed for an additional 6 weeks and then given an additional 5 weeks of training maintained the initial improvement on the verbal serial position memory tasks at the 6-week follow-up. The 5 extra weeks of training led to further improvement, which was sustained throughout a 10-week posttreatment follow-up. This patient’s mean performance on the three tests over the posttraining follow-up period was 50% higher than it was at the two pretraining baseline assessments.
Figure 1 shows normalization of this patient’s left inferior frontal cortex activation during the four-word auditory verbal serial position memory task after training. The figure also shows activation of the left inferior frontal cortex in a typical healthy subject during this task
(14). No such activation was present in the patient before training, some was apparent after 10 weeks of training, and activation was normal after 15 weeks of training. Activation of auditory areas within the superior temporal gyri were at least as great before training as after training at these thresholds, providing internal references for comparisons of frontal activation.