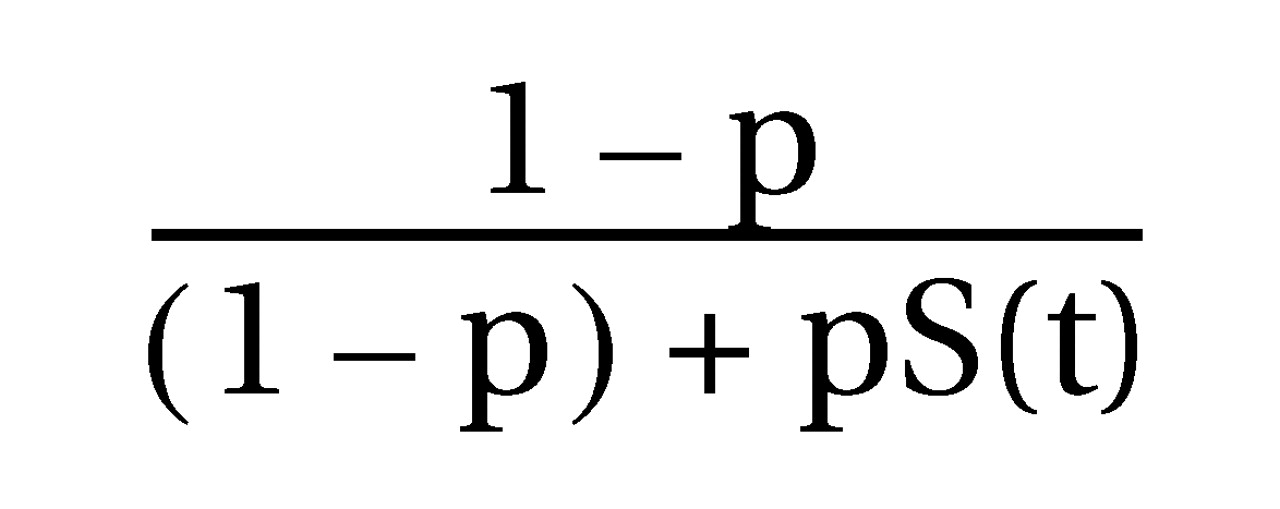The timing of onset of clinical improvement with the selective serotonin reuptake inhibitors (SSRIs) fluoxetine, sertraline, paroxetine, and citalopram, as well as with bupropion, trazodone, nefazodone, and mirtazapine, has been studied less extensively than the timing of improvement with the older generation of tricyclic antidepressants and monoamine oxidase inhibitors. Head-to-head studies of SSRIs and tricyclic antidepressants have indicated parallel improvements when measured by standard depression scales
(8–
11). In addition, on the basis of the hypothesis that combined norepinephrine and serotonin uptake inhibition causes a more rapid down-regulation of beta adrenergic receptors than with norepinephrine alone
(12), Nelson and colleagues
(13) found that combining fluoxetine and desipramine resulted in faster antidepressant effects than with desipramine alone. With a similar line of reasoning, that dual action speeds up response, a faster onset of action has been reported for venlafaxine than for SSRIs
(3).
The purpose of this report was to assess the time until response with open fluoxetine treatment and to determine the variables associated with fast and slow response. To define clinical improvement, we decided to use a 30% reduction in score on the 17-item Hamilton Depression Rating Scale
(14) at baseline, without a subsequent increase at follow-up visits, on the basis of findings by Nobler et al.
(15) and consistent with the work of Möller and colleagues
(16). Nobler et al.
(15) defined clinical improvement as a 30%, 40%, 60%, or 70% decrease in scores on the Hamilton depression scale during the course of ECT treatments; they assessed time until onset of response with right unilateral or bilateral ECT with low- or high-energy delivery on the basis of seizure threshold. The criterion of a 30% reduction in baseline scores on the Hamilton depression scale was found to best discriminate time until the onset of response between effective and less effective forms of ECT. To model time to onset, we used survival analysis because of its greater sensitivity detecting differences in the onset of response
(15) compared to linear regression and random regression models.
Method
The initial study group consisted of 384 outpatients (210 women, 55%) between the ages of 18 and 65 (mean age=39.9 years, SD=10.5) who met the criteria for major depressive disorder as measured by the Structured Clinical Interview for DSM-III-R—Patient Version
(17) and who had a 17-item Hamilton depression scale score of 16 or higher at baseline. None of the subjects entering the study had failed to respond to an adequate antidepressant trial during the current episode. The purpose of this parent study was to generate a number of prospective nonresponders to fluoxetine for further study of options to induce response in nonresponders. Patients who entered the open trial received a fixed dose of 20 mg/day of fluoxetine for 8 weeks. The Hamilton depression scale was administered at baseline and then every 2 weeks to assess depression severity. Patients met every 2 weeks with physicians to discuss the benefits of medication, side effects, and adverse events. The study was conducted by the Depression and Clinical Research Program at Massachusetts General Hospital, and the study protocol was approved by Massachusetts General Hospital’s institutional review board. All subjects had to be able to understand the written informed consent statement and sign it voluntarily. Rights of confidentiality were reviewed. After complete description of the study to the subjects, written informed consent was obtained.
Exclusion criteria included having a history of organic mental disorders, history of seizure disorder, serious or unstable medical illness, substance use disorder (including alcohol) within the last 6–12 months, serious suicidal risk, pregnancy, lactation, schizophrenia, delusional disorder, psychotic disorders not elsewhere classified, mood congruent or mood-incongruent psychosis, bipolar disorder, significant antisocial personality disorder, history of multiple adverse drug reactions or intolerance of or nonresponse to study drugs, concomitant use of nonstudy psychotropic drugs, and clinical or laboratory evidence of hypothyroidism.
Treatment response was defined as a 50% decrease in score on the Hamilton depression scale from baseline to endpoint; remission was defined as a final Hamilton depression scale score of 7 or lower
(18,
19). Time until onset of response was defined as the first time point at which the score on the Hamilton depression scale decreased by 30% from baseline without a subsequent increase. By including only those without any increase in Hamilton depression scale scores, we excluded patients who had a placebo pattern of nonsustained response
(20). The responding group represented the best-case scenario: group members had a true drug pattern of response and responded or experienced remission by the end of the 8-week trial. The rationale for segregating responders was that if nonresponders were included in the same group, the time until onset of response would be delayed because of a reduced overall response rate and would lead to a false conclusion about the time until response for responders
(21).
Survival analysis
(22) was used to assess the time until onset of response and the time until response. The probability of never having an onset of response was calculated according to the methods of Laska and Siegel
(21) by using the formula
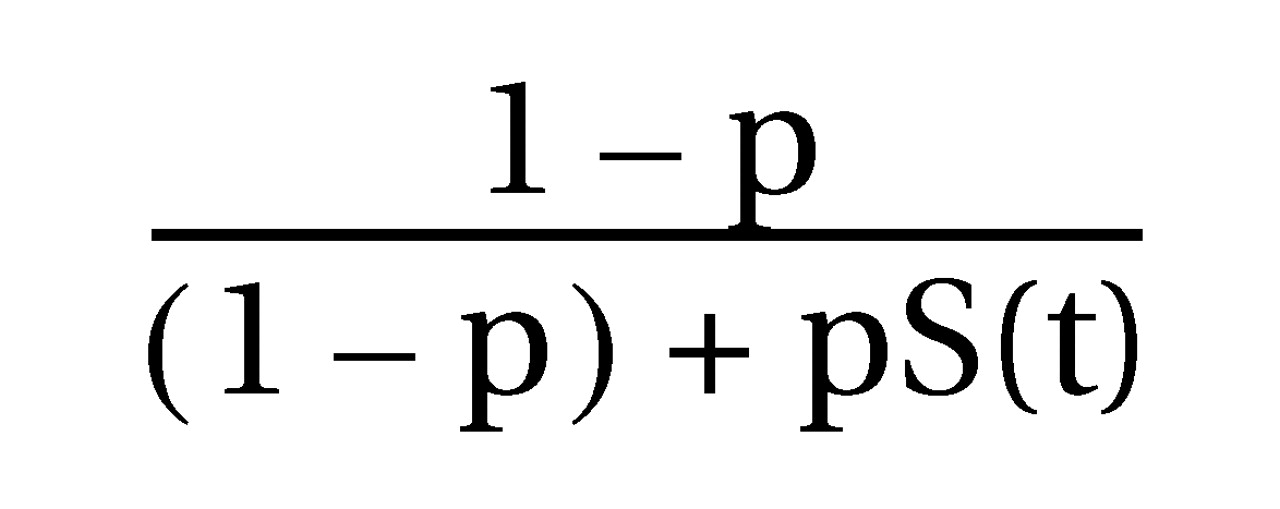
in which “p” is the proportion of the group with a response and “S(t)” is the cumulative probability that the time to onset of response is greater than “t” among patients with a response. In contrast, the more conventional measure is “H(t),” the cumulative probability that the time until onset of response is greater than “t” among all patients, including those without a response. Cox regression analysis for proportional hazards was used to model the covariates of the time until onset of response and the time until response. Chi-square analysis and unpaired t tests were used for categorical and continuous variables, respectively, to assess differences between the group with a persistent response and the remainder of the patient group.
Results
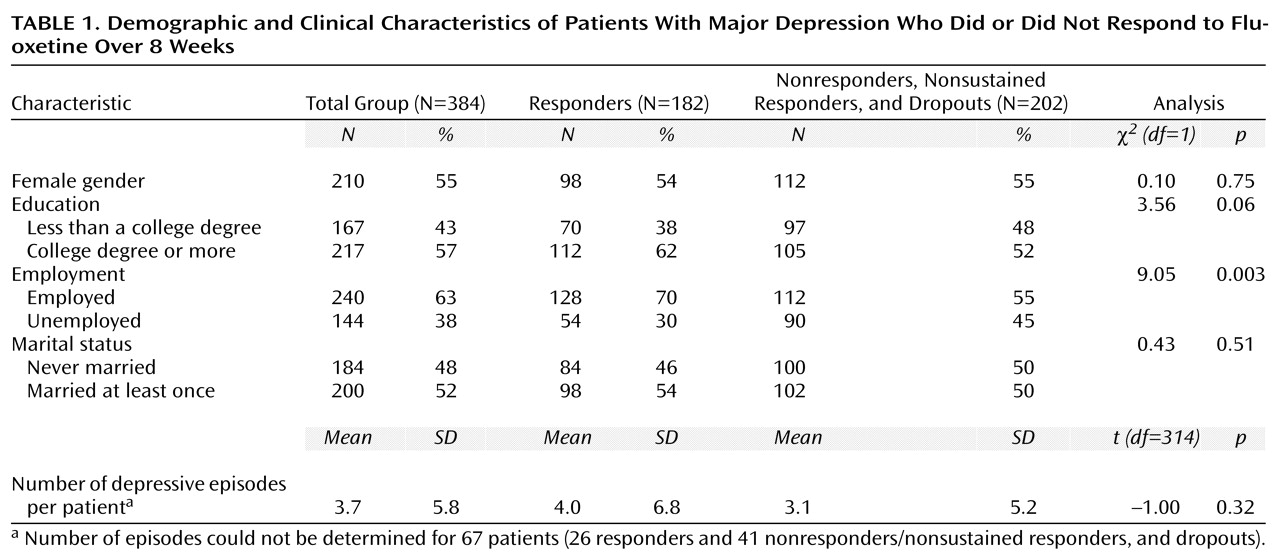
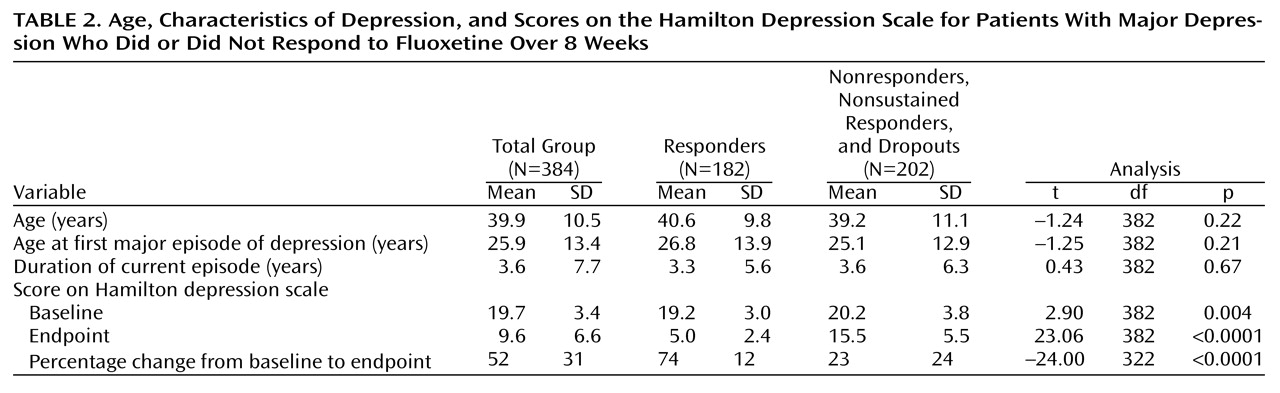
A total of 324 (84.4%) of the 384 patients in the study completed the open trial; there were 60 dropouts (15.6%). Of the 384 patients, 193 (50.3%) responded, and 148 (38.5%) had acute remission with final Hamilton depression scale scores of 7 or lower. (Of the 193 responders, 148 [76.7%] experienced remission of their symptoms. Of 324 who completed the study, 193 [59.6%] responded, and 148 [45.7%] experienced remission of their symptoms.) A total of 182 (94.3%) of the 193 patients who met the criteria for response were included in the responder group; the criteria were all data points present, a 30% decrease in baseline score on the Hamilton depression scale without subsequent exacerbation, and a 50% reduction in baseline score on the Hamilton depression scale after 8 weeks of treatment with 20 mg/day of fluoxetine. The response group consisted of 98 (54%) women, with an overall mean age of 40.6 years (SD=9.8) and a baseline mean Hamilton depression scale score of 19.2 (SD=3.0). Responders had a mean posttreatment Hamilton depression scale score at 8 weeks of 5.0 (SD=2.4) and a mean percentage decrease in score from baseline of 74% (SD=12%). The mean duration of the current episode of depression for responders was 3.3 years (SD=5.6). A total of 206 (57%) of the 360 patients with evaluable durations of the current episode of depression had durations of less than 2 years. The mean age at onset of the first depressive episode was 26.7 years (SD=13.9). Differences in demographic and baseline clinical variables between sustained responders (N=182) and the group of nonresponders, dropouts, and nonsustained responders (N=202) were not statistically significant (
Table 1 and
Table 2), except that the sustained responders had slightly lower baseline Hamilton depression scale scores and were more likely to be employed.
We found that at weeks 2, 4, and 6, when the entire group of patients was assessed, 26.0%, 19.7%, and 11.7% of the patients, respectively, showed an onset of response as defined as a sustained 30% decrease in baseline score on the Hamilton depression scale that led to a final decrease of 50%. Cumulative probabilities of onset of response at each time point were 26.0%, 45.7%, and 57.4%. Alternatively, time until response with the criterion of a 50% decrease in baseline score on the Hamilton depression scale showed probabilities of response at weeks 2, 4, and 6 of 10.6%, 21.8%, and 16.0%; cumulative probabilities of response at the time points were 10.6%, 32.4%, and 48.4%, respectively, and 61.4% at week 8.
When responders were assessed with life table analysis, 55.5%, 24.7%, and 9.3% had an onset of response at weeks 2, 4, and 6; cumulative response rates at each time point were 55.5%, 80.2%, and 89.5%
(21). Mean time to onset of response was 3.8 weeks. Alternatively, the portions of the group to respond (response defined as a 50% decrease in baseline score on the Hamilton depression scale) were 18.1%, 36.8%, and 26.4% for weeks 2, 4, and 6; cumulative probabilities of response were 18.1%, 54.9%, and 81.3%. Mean time to response was 4.9 weeks. The difference between the mean time to onset of response and the mean time to response was 1.1 weeks. With the use of completer analysis, we found that the probability of a patient never having an onset of response, given that onset had not occurred by weeks 2, 4, or 6, was associated with the probability of never having an onset of response (55%, 73%, and 88%, respectively). Neither demographics (age and sex) nor characteristics of depression (duration of current episode, number of episodes, age at onset of first episode, and baseline score on Hamilton depression scale) predicted time to initial response or time to response by Cox regression analysis for proportional hazards.
Discussion
The results of this study suggest that in a best-case scenario, more than half of the eventual responders to a fixed dose of fluoxetine will start to respond to treatment by week 2, with over 75% starting to respond by week 4. No predictors of time to response were found. These data show that if patients have not experienced an onset of response by weeks 4 or 6, they have about a 73%–88% chance of not exhibiting an onset of response by the end of the 8-week trial. We previously reported that nonresponders at weeks 4 and 6 were less likely to become responders at week 8, whereas early responders at weeks 2 or 4 were more likely to be responders at week 8
(23). The current study extends these findings from a totally different study group to model the time until onset of response rather than just the time until response.
Previous studies of the time until onset of response revealed variable results because of differences in antidepressants studied and methods applied. Katz and colleagues
(2) compared the onset of response between imipramine and amitriptyline and noted that patients’ anxiety, cognitive impairment, and depressed mood improved in the first week, at least for those patients who recovered by the end of the antidepressant trial. Although Katz and colleagues
(2) postulated that imipramine caused neurochemical changes within the first week of treatment, the clinical relevance of these changes, outside of the sedation caused by antihistaminergic effects, were questionable. Most theories of antidepressant action now postulate that immediate uptake inhibition of catecholamines and/or indoleamines initiates adaptive responses in pre- and postsynaptic receptors, followed by subsequent changes in proto-oncogenes and brain-derived neurotrophic factors. These final changes occur only after long-term (i.e., weeks) of exposure to antidepressants
(4,
24).
Determining the onset of response to antidepressants is further complicated by the placebo effect. Quitkin and colleagues
(25) assessed the onset and persistence of clinical improvement. Their patients were randomly assigned to receive placebo, imipramine, desipramine, phenelzine sulfate, or mianserin hydrochloride in multiple randomized clinical trials and were assessed with the Clinical Global Impressions (CGI) scale. Results showed a difference between placebo and true drug patterns of response when persistent response was considered. True drug effect was noted to occur by the 21st day of treatment, whereas the placebo effect occurred within the first 2 weeks. In a follow-up study, Quitkin and colleagues
(26) compared placebo and true drug response and found that true drug response was characterized by a 2-week delay in onset of response and persistent improvement.
Another study by Quitkin and colleagues
(27) associated anticipation of help and sudden improvement with response to placebo. Those with gradual improvement with placebo may have had spontaneous remission. Those with sudden onset of response with placebo had a tendency to lose their response within the first few weeks or had a response that was inconsistent from week to week. Quitkin and colleagues
(20) suggested that the neurophysiologic process that accounts for early abrupt improvement is the same for those with a placebo-pattern response to a drug and those who respond to placebo. Both an early placebo effect and a specific drug response occur in drug treatment, and gradual improvement in drug treatment includes spontaneous remission as well as a true drug response.
In contrast to the findings of Quitkin and colleagues
(20,
27), Stassen and colleagues
(28,
29) as well as Möller and colleagues
(16,
30) and Müller and Möller
(31) found that an early response to antidepressants was a robust predictor of sustained improvement. Müller and Möller
(31) pointed out the difficulties in comparing studies at the onset of response because of the heterogeneity of methods and definitions of response. No studies could be found, however, that assessed variables associated with time until response.
In addition, there are methodological and conceptual challenges in modeling time until onset of response. Limited consensus exists for the definition of the onset of antidepressant response because of the heterogeneity of rating scales and the statistical analyses employed
(3,
21,
32,
33). For example, self- and therapist-rated scales have differed in specificity and sensitivity, leading to limited conventional means to define onset of response. It has been noted that patients perceive improvement later than do therapists, and of course, the onset of response is directly related to the definition of response. Stassen and colleagues
(28) defined the onset of response as a 20% reduction in score on the 17-item Hamilton depression scale without a subsequent increase in score. Their findings suggested that this definition was predictive of maintenance of improvement. Angst and colleagues
(19) required a 50% reduction in baseline depression measures to define onset of response, plus a cutoff Hamilton depression scale score of less than 10. The criterion of a 50% reduction of baseline severity has been frequently used. Montgomery
(33) suggested, however, that a 50% reduction was too insensitive and that a 25% reduction in baseline severity be used, with the rationale that 25% is half of 50%. In an earlier study, Montgomery
(33) used a decrease of 4 points in score on the Montgomery-Åsberg Depression Rating Scale to define onset of response. Others have used the CGI and other rating scales. We used a 30% decrease in baseline score on the Hamilton depression scale to define onset of response, informed by the work of Nobler and colleagues
(15).
The time until onset of response is dependent on the selected response criteria. Stricter response criteria are associated with a later onset of response
(31,
34). Möller and colleagues
(16) reanalyzed data from clinical trials of brofaromine compared to imipramine. By asking, “When did you first feel better?,” they directly rated patients’ reports of onset of response; they also used the von Zerssen Adjective Mood Scale. The Hamilton depression scale was used as a therapist-rated measure. By using a decrease of 33%, 50%, or 60% from baseline scores on the Hamilton depression scale and the von Zerssen Adjective Mood Scale as response criteria, the investigators highlighted slight changes in response criteria that led to differences in time until onset. As one would expect, the greater the decrease in Hamilton depression scale score to define response, the slower the time until onset. The patients’ global ratings of time until onset had a correlation coefficient of r=0.61 when baseline scores on the Hamilton depression scale decreased 33%
(16), which is a finding similar to that of Katz et al.
(2) and Stassen and colleagues
(28,
29). Stassen and et al. indicated that early onset is a reliable predictor of maintenance of improvement.
How clinicians should apply these data to clinical practice is anything but straightforward. If one asserts that an antidepressant trial of fluoxetine at a fixed dose of 20 mg should be 8 weeks long, then if no response is detected by the sixth week, our data suggest that it is highly unlikely that a response will occur within the next 2 weeks. The dose should be increased, the drug should be augmented with another drug, or the patient should be switched to another drug. On the other hand, if one asserts that an adequate trial is 12 weeks long, then these data are not applicable. We do not know if these data are generalizable to other SSRIs or to non-SSRI antidepressants.
The results should be tempered by the following study limitations: treatment was open, without blinding of the subjects or the evaluators; no placebo control group was included; measurements of improvement were made biweekly, and the time until onset could have been sooner than that interval; the Hamilton depression scale may have been insufficiently sensitive to measure the changes in depression associated with the time until onset of response; a fixed dose of 20 mg/day of fluoxetine was given for 8 weeks; and the study used fluoxetine only, and its results and may or may not be generalizable to that of other antidepressants. Nevertheless, these results may have ecological validity in open clinical practice. The study was originally designed to generate a group of nonresponders to fluoxetine for further random assignment, not to evaluate the time until onset of response in the open phase of treatment. The study design could be viewed either as a limitation (e.g., onset of response was not assessed frequently enough) or a strength (e.g., raters were not biased for response at any particular time point). Finally, almost half of this group of patients were chronically depressed (2 years or more), with a mean duration of 3.3 years (SD=5.6) for the current depressive episode. Although regression analysis showed that the length of the current episode did not predict time until onset of response, time to response, or time to remission, it is possible that this group of patients was skewed to be more chronically ill than what would be typically encountered in clinical practice.