The working memory model involves a central executive component that handles several executive functions, including planning, strategy selection, and attentional control
(1). We have previously shown
(2) that performance of a random generation task, which relies heavily on the executive process of response selection, is impaired in patients with schizophrenia. The random generation task consists of producing a long sequence of random responses (e.g., numbers) at various speeds while attempting to avoid stereotyped sequences, immediate repetitions, or a particular retrieval strategy. It combines the two conflicting requirements of answering promptly and inhibiting any response that does not comply with randomness criteria. In this task, attentional load is a function of response pacing: subjects deviate from randomness when their response rate increases, which reflects a limited control capacity
(1).
Anatomical-functional correlates of this executive control deficit have not been investigated. Studies using brain imaging to examine high-order executive processes attributed to central executive function in normal subjects point to anterior cerebral regions. For instance, on-line monitoring of performance has involved the anterior cingulate
(3), a region that is activated during a number of tasks
(4). However, to our knowledge, no covariation between the efficiency of control processes and regional activations has been observed.
To investigate the cerebral correlates of impairments in executive control processes, we examined the covariation between regional activation and performance in patients with schizophrenia and healthy comparison subjects while they performed a graded random number generation task.
Method
Eight outpatients aged 19 to 44 years (mean=28, SD=6) who met DSM-IV criteria for schizophrenia and eight healthy volunteers aged 20 to 31 (mean=25, SD=3) were recruited. All were right-handed men. Patients had a chronic course and were stabilized with neuroleptic treatment. They had marked negative symptoms: their mean score on the Scale for the Assessment of Negative Symptoms
(5) was 63 (SD=1), and their mean score on the Scale for the Assessment of Positive Symptoms
(5) was 24 (SD=13). After complete description of the study to the subjects, written informed consent was obtained from all who participated.
Twelve measurements of regional cerebral blood flow were performed with the H215O method (9-mCi injection) over a 2-hour period (scan duration=80 seconds; interscan duration=10 minutes). Positron emission tomography scans were acquired on a Siemens HR+ tomograph (Knoxville, Tenn.) (63 slices; 2.4-mm thickness; 128×128 pixels of 2.4 mm), with measured attenuation correction (10-minute 68Ge/68GA transmission scan).
The experimental task was random number generation; the baseline was iterative counting of numbers. Both involved oral production of numbers between 1 and 10 inclusive, guided by computer-generated tones at the rates of one tone every second, 2 seconds, or 4 seconds. The order of tasks was counterbalanced, and each rate of generation/counting was run twice, so that each subject performed 12 randomized runs (two tasks × three rates × two scans).
The number of responses and the random number generation index, a measure of deviation from randomness computed on the basis of the frequency of successive identical pairs of numbers
(6), were determined from each subject’s tape recordings. A repeated measures analysis of variance (ANOVA) with a group factor (healthy subjects versus patients) and a rate factor (one number per 1, 2, or 4 seconds) was performed.
We examined covariations between the random number generation index and cerebral activation for each rate, using statistical parametric mapping (SPM99b software, Wellcome Department of Cognitive Neurology, London). We used analysis of covariance to test for the interaction between regional activation (random number generation minus counting of numbers) and the random number generation index. Because the random number generation index is assumed to relate negatively to executive control
(6) (i.e., the lower the random number generation index, the better the control), a negative modulation of the activation (random number generation minus counting of numbers) by the random number generation index was examined in each group and between groups. The significance level was p=0.001 uncorrected for the height threshold; the extent threshold was 250 voxels (2 cc).
Results
Patients’ performance was impaired. Regarding the number of responses, ANOVA showed an effect of group (F=6.49, df=1, 14, p<0.03) and rate (F=564.20, df=2, 28, p<0.0001) as well as a group-by-rate interaction (F=5.96, df=2, 28, p<0.03). Fewer patients than normal subjects responded at the fastest rate (one per second). Random number generation index analysis revealed an effect of group (F=6.24, df=1, 14, p<0.03) and rate (F=64.70, df=2, 28, p<0.0001) but no group-by-rate interaction (F<1, df=2, 28, n.s.).
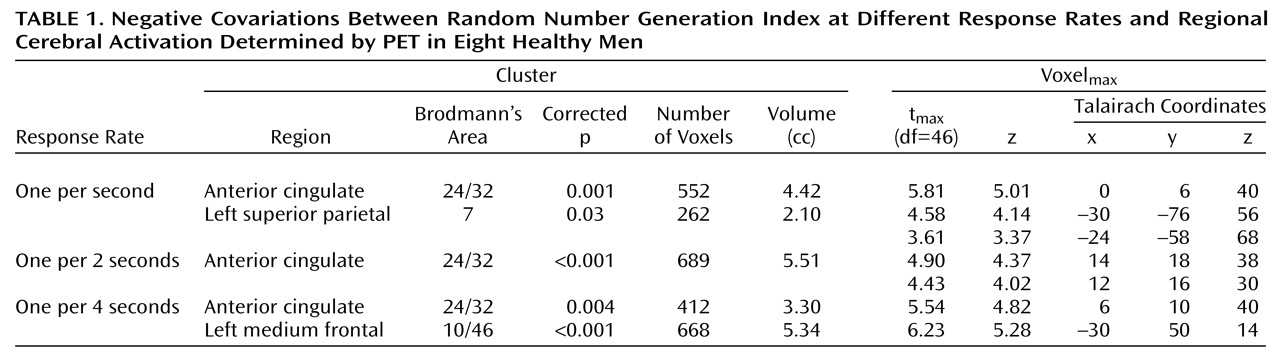
In healthy subjects (
Table 1), for each rate, activation covaried negatively with random number generation indexes in the anterior cingulate (Brodmann’s area 24/32). In patients, no activation covaried with the random number generation indexes. The between-group comparison (
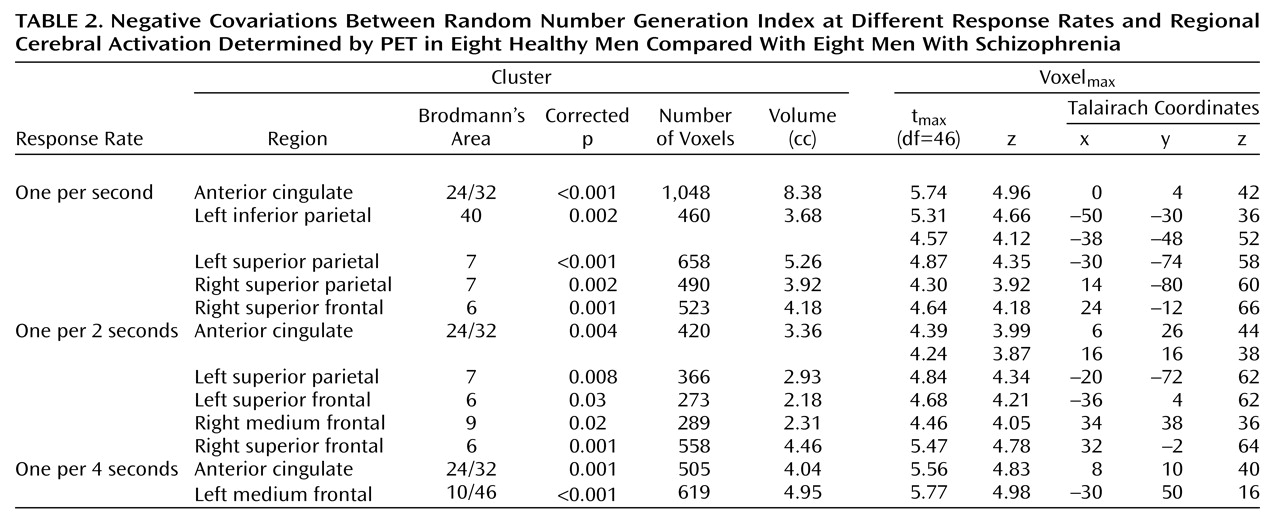
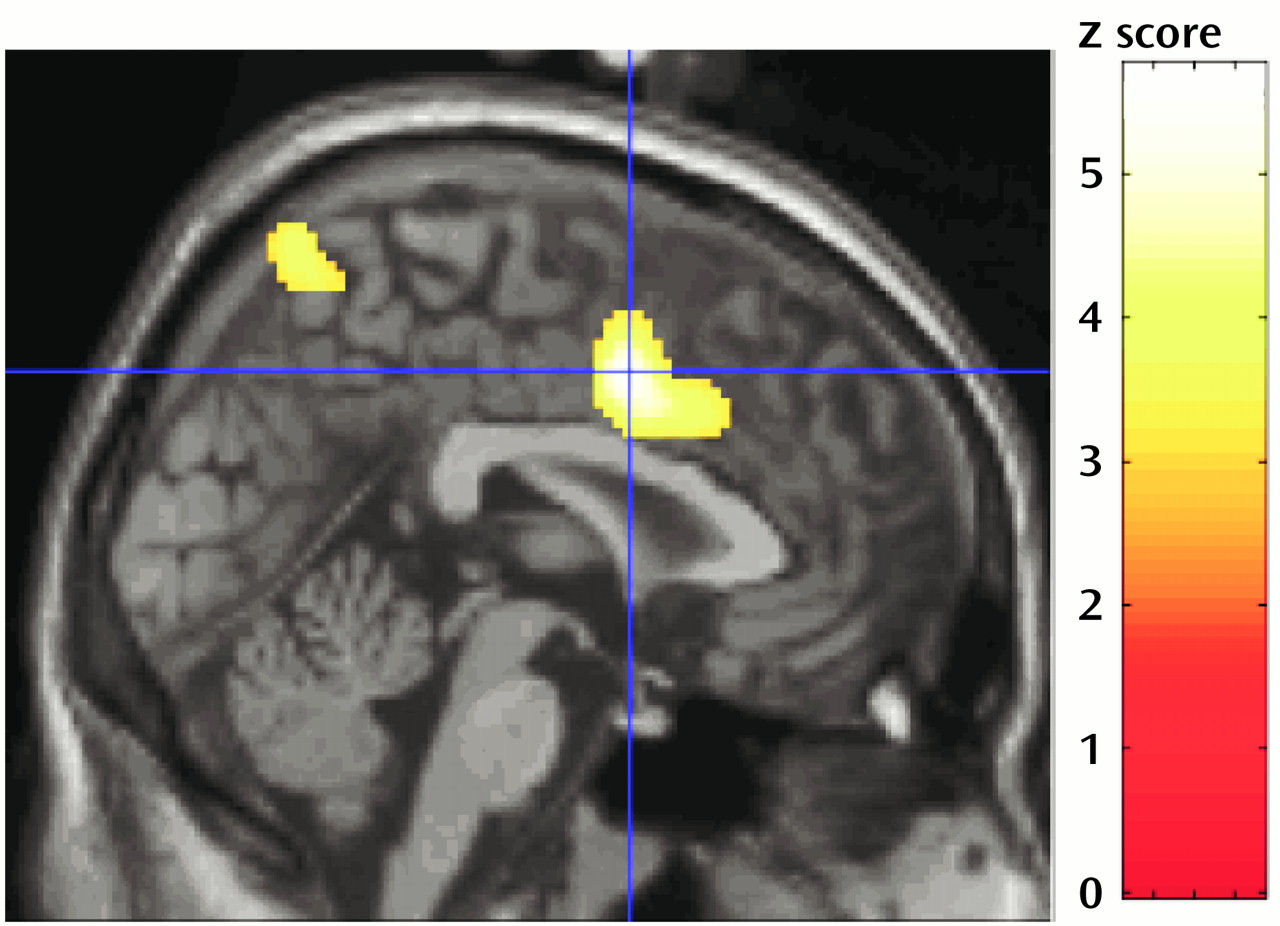
Table 2 and
Figure 1) showed the regions where the covariation between random number generation index measures and regional activation (random number generation minus counting of numbers) differed significantly between patients and normal subjects. Thus, in the superior anterior cingulate, between-group differences were found across the three task difficulty levels, and marked differences in parietal regions depended on pacing and were detected only at one per 2 seconds and one per second response rates.
Discussion
Randomness of responses, as measured in a random number generation task, was impaired at all rates in patients and increased as a function of rate in all subjects, consistently with our previous report
(2).
In patients, no covariation was found between activity in the anterior cingulate and the ability to carry out the task properly (i.e., random number generation index values). In healthy volunteers, the search for modulation of regional activations by random number generation index consistently involved the superior anterior cingulate: the better the randomness of responses, the greater the activation. Therefore, the anterior cingulate appears to be an essential structure to executive control. This suggests a link between the superomedial anterior cingulate and a controlled process of response selection that plays a role in random generation. Indeed, the subject must continuously select a new response while avoiding the automatic tendency to produce predetermined, or stereotyped, sequences. Beyond the random generation task itself, the process of response selection and the activation of the superior anterior cingulate are critical in a number of cognitive tasks
(4). Therefore, our results support the proposition that alteration of anterior cingulate processes might account for several aspects of the cognitive impairments exhibited by patients with schizophrenia.
The absence of a correlation between the random number generation index and activation in the superior parietal cortex in patients at the fastest rates may denote an impaired adaptation to gradual difficulty in number processing. Superior parietal activity also increases with difficulty in other working memory tasks
(7).
Our finding of a cinguloparietal dysfunction provides further evidence of the impaired central executive control process of response selection in patients with schizophrenia. Further investigations are needed to examine whether this dysfunction is related to symptoms of schizophrenia such as loose associations, scrambled language, disordered thinking, and formal thought disorder
(8).