Clozapine, unlike typical antipsychotics, does not elevate prolactin levels
(1). This unique clinical feature led researchers to classify hyperprolactinemia as one of the hallmark features of “atypicality”
(2). Newer antipsychotic medications that mirror this profile have been developed
(3,
4). Risperidone is the exception in that it does not mirror the profile of clozapine
(5).
A major limitation of the clinical studies of the atypical antipsychotics is that data derive from prolactin measurements at a single point in time, usually 12–24 hours after medication administration. There is evidence that atypical antipsychotics have some effect on the prolactin system. For example, it has been demonstrated that the acute administration of clozapine leads to rapid, short-lived prolactin elevation
(6).
This study is an attempt to explore whether administration of three commonly prescribed atypical antipsychotics resulted in dose-related transient elevation of prolactin levels in patients with chronically treated schizophrenia.
Method
The study was approved by the Human Subjects Review Committee of the University of Toronto. Written informed consent was obtained from all participants after the procedures had been fully explained. Male patients with a diagnosis of schizophrenia were included if they were taking either clozapine (at least 300 mg/day), risperidone (1–6 mg/day), or olanzapine (10–20 mg/day); if they had been receiving these medications for more than 8 weeks; and if the prescribed dose had remained the same for at least 1 week.
Patients were excluded from participation if they had been prescribed depot antipsychotics within 6 months of the study; had any physical condition that could affect prolactin levels such as endocrine disorders; or were receiving concomitant antidepressant or antiparkinsonian medication. Other concomitant medications that are not known to affect the prolactin-secreting system were permitted; however, the patients did not take any of these drugs on the day of the study. Subjects were asked to fast and not take their evening antipsychotic medication the night before the study.
On the day of the study, subjects arrived at the hospital at approximately 8:00 a.m. At that time an indwelling intravenous catheter was inserted into the subject’s forearm vein for repeated blood sampling. After 30 minutes, two baseline prolactin samples were obtained 15 minutes apart. Once the second baseline sample was obtained, subjects were instructed to take their medication. Blood samples were then obtained every 60 minutes over an 8-hour period. Subjects returned the next day so that a final blood sample could be obtained at 24 hours.
Blood was centrifuged in glass tubes, and plasma was stored in plastic tubes at –80°C until prolactin assays were completed. Plasma prolactin levels were determined by using microparticle enzyme immunoassay technology with a minimum detectable limit of 0.6 ng/ml and a coefficient of variation of 4.5% (St. Joseph’s Health Centre, London, Ont.). The upper limit of normal for plasma prolactin of 20 ng/ml for men during the waking state was based on sample of 85 healthy male adults (Abbott Laboratories, Abbott Park, Ill.).
Subjects were also given the option of returning and repeating the entire procedure on a separate occasion without taking their antipsychotic medication. This was done so that they could act as their own drug-free control subjects. Five subjects agreed and returned at least 1 month after their initial visit.
Results
Eighteen male patients with a mean age of 32 years (SD=8) entered the study. Median doses for each drug group were as follows: olanzapine (N=6), 20 mg (range=10–20 mg); risperidone (N=6), 3 mg (range=1–3 mg); clozapine (N=6), 300 mg (range=300–400 mg). The median duration of current antipsychotic treatment was 12 months for patients receiving olanzapine, 36 months for those receiving clozapine, and 8 months for those receiving risperidone.
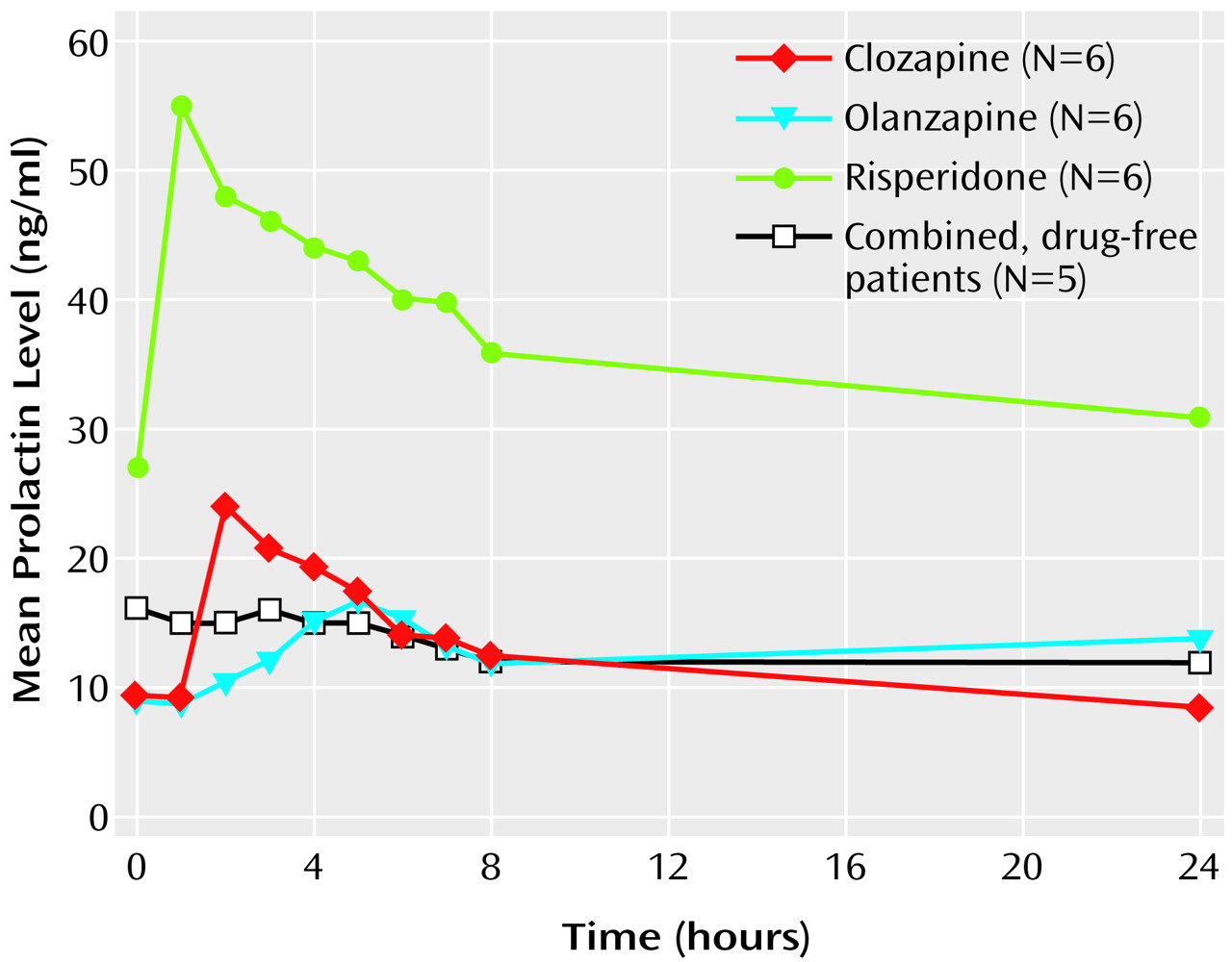
There was a statistically significant difference in mean baseline plasma prolactin levels among the three medications. The baseline plasma prolactin level of patients receiving risperidone was 27 ng/ml (SD=14), compared with 9 ng/ml (SD=5) for those receiving olanzapine and 9 ng/ml (SD=5) for those receiving olanzapine (F=11.43, df=2, 15, p<0.01). Three of the six patients receiving risperidone were hyperprolactinemic, but the prolactin levels of all patients receiving olanzapine or clozapine were within normal limits.
A repeated measures analysis of variance applying a cubic trend model revealed a significant difference among drugs (F=27.54, df=2, 15, p<0.01), a significant time effect (F=11.62, df=1, 15, p<0.01), and a significant drug-by-time interaction (F=4.90, df=4.88, 36.66, p<0.02) (
Figure 1).
The drug-by-time interaction was explored by using simple effects analysis examining the polynomial contrasts of time at each drug level. This analysis revealed a significant elevation in prolactin level induced by clozapine (F=3.74, df=9, 135, p<0.001) and risperidone (F=6.26, df=9, 135, p<0.001) but not olanzapine (F=1.24, df=9, 135, p>0.05).
There was a statistically significant difference in time to reach peak prolactin levels between risperidone (mean=120 minutes, SD=53), olanzapine (mean=290 minutes, SD=45), and clozapine (mean=180 minutes, SD=53) (F=17.15, df=2, 15, p<0.001).
Within-subject control data were obtained for five patients (one receiving clozapine, two receiving risperidone, and two receiving olanzapine). These patients repeated the procedure of blood draws and prolactin level measurements at least 1 month after their initial visit without taking their usual dose of risperidone, olanzapine, or clozapine. Each patient demonstrated only minor prolactin fluctuations (
Figure 1). There was a significant difference for each patient in prolactin levels over the course of 24 hours after taking the medication and after not taking the medication (F=3.51, df=9, 36, p<0.01).
Discussion
These results indicate that clozapine and risperidone administration results in dose-related elevation in prolactin levels, even after patients have been taking these drugs for a prolonged period of time. Patients receiving olanzapine also showed a near doubling over baseline of prolactin levels, from mean=9 ng/ml (SD=5) to mean=18 ng/ml (SD=10). This difference was not significant, however, presumably because there were only six patients in the group and there was a higher variance in this group. More importantly, our study shows that the increases in prolactin levels with atypical antipsychotics are most pronounced in the 1–5-hour period after medication administration and return to baseline values by 12–24 hours, thus masking the drug’s acute effect on prolactin. Risperidone is the exception to this pattern.
The study is limited by the fact that it was restricted to male patients, the number of patients studied was only 18, standard deviations were large, the patients were not randomly assigned to receive the different drugs, and not every patient participated in the second prolactin measurement after not taking their medication. Nonetheless, we can be reasonably certain about the central conclusion: even atypical antipsychotics give rise to transient drug-specific elevations of prolactin levels, and the difference between typical antipsychotics and atypical antipsychotics has more to do with the degree and duration of prolactin elevation than to a categorical difference.
The precise mechanisms whereby use of atypical antipsychotics leads to a transient effect on pituitary dopamine D
2 receptors are unclear. It has been shown that for quetiapine
(7), the transient prolactin elevation corresponds to transient dopamine D
2 receptor blockade. Whether this is the case for other atypical antipsychotics needs to be confirmed. Further, although the adverse consequences of continuous prolactin elevation are well documented, whether this transient elevation has any clinical implications should be the subject of future investigations.