Schizophrenia is characterized by abnormalities in multiple functional systems, including perception, attention, cognition, and arousal. Many efforts to define the neuropathological substrates of schizophrenia have sought to associate particular symptoms with specific regions of the brain. However, some constellations of schizophrenia’s functional deficits might be better explained by a lesion in an area in which the relevant circuits converge, such as the thalamus
(1). Since diverse brain regions are interconnected via the thalamus
(2,
3) and because pathology in one brain region may induce pathology in other regions with which it communicates
(4), thalamic pathology may hold a key to understanding why multiple brain regions exhibit schizophrenia-associated structural, cellular, and molecular anomalies
(5–
8).
Thalamic abnormalities in schizophrenia have been documented by several
(9–
16) but not all
(17,
18) studies through both in vivo neuroimaging and postmortem morphometric techniques. The most consistent autopsy finding in the thalamus has been lower volume
(10–
13) and cell number
(10–
14) in the mediodorsal nucleus. These changes, which have not been detected by some studies
(17,
18), have been reported to affect the left- and right-sided mediodorsal nucleus equally and to be independent of neuroleptic exposure
(11). One study reported neuronal loss in the mediodorsal nucleus to be restricted to its lateral subdivisions, which have a predominance of projections to the dorsolateral prefrontal cortex, in contrast to its medially situated magnocellular subdivision, which largely has projections to the orbital and medial aspects of the frontal lobe
(14). Relatively few studies have examined other thalamic nuclei for schizophrenia-associated changes
(9,
13–
16), so the specificity of such changes to the mediodorsal nucleus has not been thoroughly assessed. In a magnetic resonance imaging study of living subjects, we recently detected schizophrenia-associated lower volume in the pulvinar in addition to the mediodorsal nucleus
(9). The significance of this finding may be that the mediodorsal nucleus and pulvinar are the largest association nuclei of the thalamus and that both have extensive connections with the dorsolateral prefrontal cortex and other brain regions consistently implicated in schizophrenia. A lower number of neurons has also been reported in the anterior thalamic nucleus (13, 15) in the absence of volume reduction
(13). A lower number of neurons in the pulvinar
(16) and periventricular tissue loss (Treff [1958], cited in reference
10) have also been described. At least three previous studies, which noted cellular changes in the mediodorsal nucleus or pulvinar, failed to detect abnormalities in more laterally situated nuclei
(9,
16).
In the present study, we examined the volumes of the mediodorsal nucleus and pulvinar in a postmortem cohort of schizophrenic subjects and nonpsychiatric comparison subjects. Two of the most well-demarcated thalamic nuclei, the centromedian and anterior nuclei (as delineated by Feremutsch and Simma for the Dewulf human thalamic atlas
[19]), were chosen as comparison regions to test the specificity of volume differences to the mediodorsal nucleus and pulvinar. In addition, we counted and measured neurons in the mediodorsal nucleus and pulvinar to see if lower volume was associated with smaller neuronal size or lower number. The subdivisions of the mediodorsal nucleus were examined separately to determine whether they were differentially affected. Our hypotheses were that 1) volume differences would be seen in both the mediodorsal nucleus and pulvinar but not in the anterior or centromedian nuclei; 2) differences in neuronal count would be seen in both the mediodorsal nucleus and pulvinar; and 3) differences in neuronal count within the mediodorsal nucleus would be greatest in the parvocellular subdivision because of its extensive connections with the dorsolateral prefrontal cortex, a region consistently implicated in schizophrenia
(7,
9,
14).
Method
Specimens for this study were obtained from the Mount Sinai/Bronx VA Schizophrenia Brain Bank
(20,
21), which collects tissue in accordance with approved institutional review board protocols. After a variable postmortem interval and removal from the calvarium, right hemispheres were fixed for 10–14 days in 4% paraformaldehyde. Left hemispheres are routinely flash-frozen by the Brain Bank and so were not suitable for the present study. All specimens from the Brain Bank in which the right side of the thalamus was intact were obtained (N=25) and coded to ensure that diagnostic information was not available to the investigators involved in subsequent measurements. The entire thalamus was then dissected from the hemisphere as a single block and post-fixed in fresh buffered 10% neutral formalin so that the total fixation time was ≥20 weeks to maximize fixation shrinkage and thereby minimize variation due to shrinkage
(22). Blocks were saturated in a 30% sucrose solution at 4°C and sectioned serially at 80 microns in a coronal plane on a freezing microtome. Every 10th section with a random start between 1 and 10 was stained with thionin, yielding 17–22 stained sections per thalamus.
After processing, three specimens were excluded from further analysis because of gross lesions affecting the thalamus. The remaining group included 14 subjects with DSM-IV-defined schizophrenia and eight nonpsychiatric comparison subjects (
Table 1). Subsequent review of the neuropathological data revealed that four specimens from the schizophrenia group and three from the comparison group met minimal criteria for Alzheimer’s disease
(21). This left a total of 10 specimens from the schizophrenia group and five from the comparison group without neuropathological comorbidity (
Table 1). No specimens came from subjects with a known history of alcohol or other substance abuse, and none had documented pathology of the mammillary bodies.
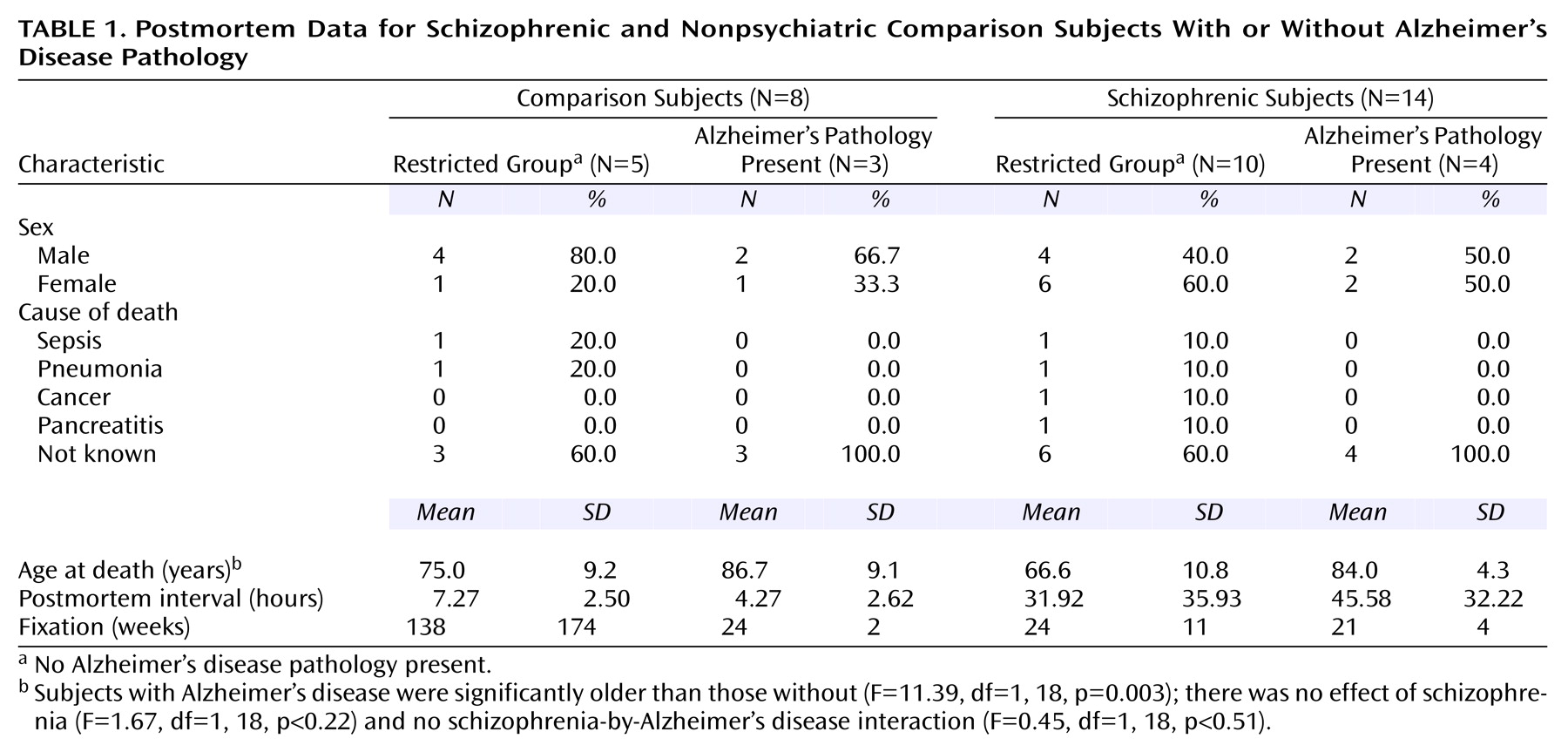
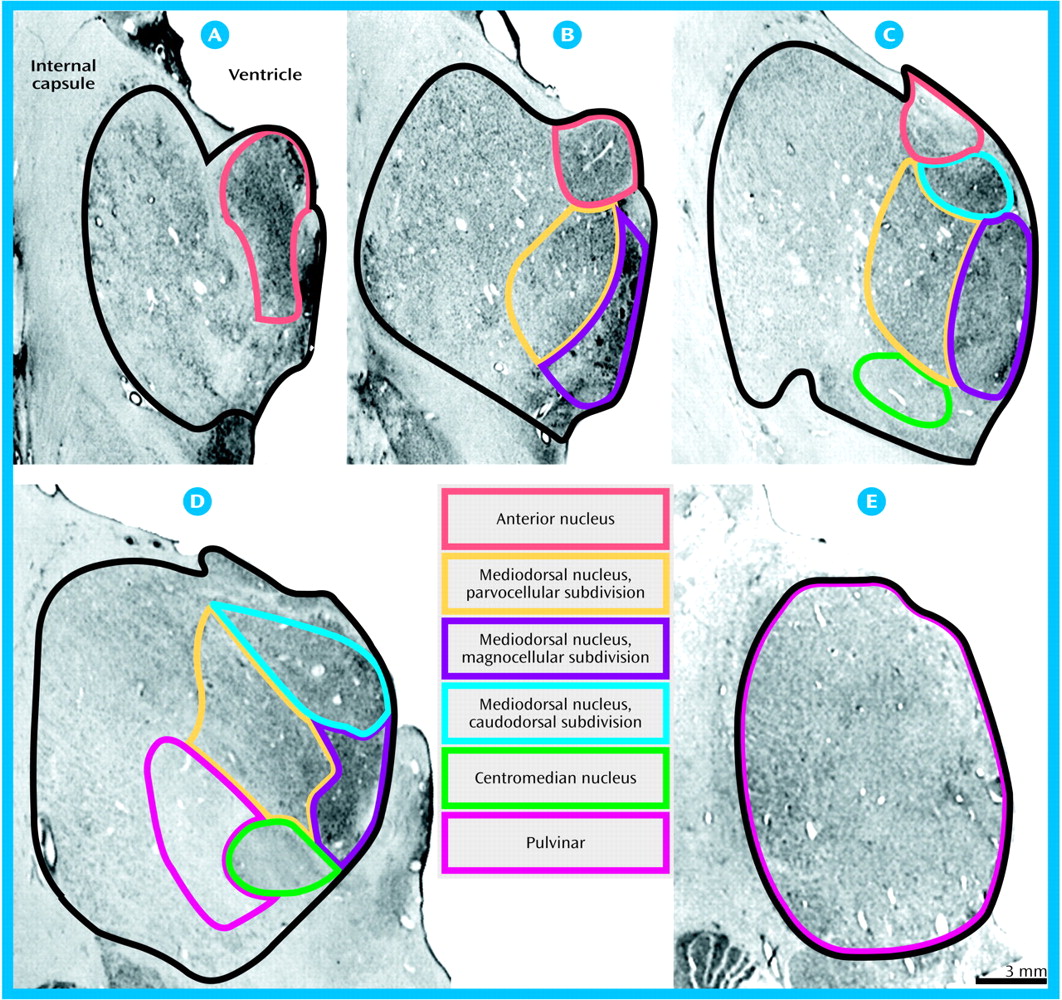
Volumes for each region of interest were assessed with a Bioquant True Colors Windows Image Analysis System (R&M Biometrics, Nashville, Tenn.). The regions of interest were the total thalamus, the mediodorsal nucleus (and its parvocellular, magnocellular, and caudodorsal subdivisions), the pulvinar, and the anterior and centromedian nuclei. The mediodorsal nucleus may be parcellated into subnuclei according to various schemes (e.g., references
3,
14,
23–
25). Our delineation and nomenclature of the subnuclei followed that of Ray and Price
(24). Each thalamic section was placed on a light box and its image relayed at a final magnification of 16× to a 17-inch color video monitor by a 3-chip video camera fitted with a macro lens (10–70 mm 1:2.1). The perimeter of each region of interest was traced on each section in which it appeared with the computer cursor, and volumes were determined by the Cavalieri method
(26). All volume measurements were made in duplicate by a single investigator (W.B.) with an interval of 4 to 12 weeks between successive measurements and statistical analyses performed on the average. High intraclass correlations for the total thalamus (0.96), mediodorsal nucleus (0.92), magnocellular subdivision (0.87), parvocellular subdivision (0.88), caudodorsal subdivision (0.81), pulvinar (0.94), anterior nucleus (0.96), and centromedian nucleus (0.93) were seen for the two sets of measurements. Photomicrographs depicting delineation of the total thalamus and each region of interest are shown in
Figure 1.
The Bioquant Topographer and Stereology Toolkit (version 2.50) were used to count neurons within the mediodorsal nucleus and pulvinar at a final screen magnification of 1100×. Approximately 90 counting boxes (50 μm per side) were positioned systematically with a random start throughout the extent of the mediodorsal nucleus and pulvinar (approximately 30 fields were examined within each division of the mediodorsal nucleus). An analysis that used the method of Steel and Torrie
(27) had shown that 25 counting boxes per region of interest held counting errors at or below ±5% for both the mediodorsal nucleus and pulvinar. Cells containing visible cytoplasm and a distinct nucleolus were considered to be neurons and were counted unless their nucleoli touched the exclusion lines of the counting box (top and left edges). Neurons were counted by focusing throughout the depth of the tissue. Cross-sectional areas were determined for a minimum of 100 neurons and their nucleoli per region of interest for each specimen. Because of suboptimal cellular staining and an inability to discern nucleoli clearly, cellular analyses were not carried out on two specimens, both of which were from schizophrenic subjects without Alzheimer’s disease pathology. For calculations of neuronal densities, the depth of the counting boxes was taken as the thickness of the sections before processing (80 μm). Total neuronal number was calculated for each region of interest as the product of its volume and neuronal density. Although split cell error would be expected to be negligible, since the diameter of the objects counted (i.e., nucleoli) was small relative to section thickness, this correction was made by using formula 4 of Konigsmark
(28). For this correction, the thickness of the sections after histological processing was determined. A 100×/1.30 oil immersion objective was employed to first focus on the upper surface of each section and then on the lower surface. Section thickness was determined by subtracting the z axis coordinate of the lower surface from that of the upper surface. The corrected neuronal densities and total counts were employed in the statistical analysis.
Statistica 5.1 was employed for data analysis. Two sets of statistical analyses were done: one that included data from all subjects (full cohort) and one in which data from subjects with Alzheimer’s disease were excluded (restricted group). For the full cohort, volume data were analyzed by three-way (schizophrenia status-by-Alzheimer’s disease status-by-four regions) mixed design analyses of variance (ANCOVAs) with schizophrenia status and Alzheimer’s disease status (i.e., schizophrenia without Alzheimer’s disease pathology, schizophrenia with Alzheimer’s disease pathology, Alzheimer’s disease without schizophrenia, and presumed nonpsychiatric comparison subjects) as the grouping variables and regions (mediodorsal nucleus, pulvinar, anterior nucleus, centromedian nucleus) as repeated measures. Separate follow-up ANCOVAs were performed with the mediodorsal nucleus subdivisions (magnocellular, parvocellular, caudodorsal) as repeated measures. Age, brain weight, postmortem interval, and fixation time were entered as covariates. Although none of these variables was significantly correlated with the dependent variables at the 0.05 level, concern was raised that the lack of statistically significant correlation might be due to the small study group size. Mixed-model ANCOVAs were also employed to analyze the cellular data (neuronal size, density, and number). Since cellular data were available only for the pulvinar and for the mediodorsal nucleus and its subdivisions, the number of repeated measures varied accordingly. Total thalamic volume was analyzed by two-way ANCOVA. Planned comparisons were made by using Duncan’s multiple range test. For the restricted group, analyses were identical to those of the full cohort with the exception that there were only two diagnostic groups (schizophrenia without Alzheimer’s disease and presumed nonpsychiatric comparison subjects). All t tests were two-tailed (alpha level, p<0.05). Strictly speaking, it could be argued that one-tailed tests have been used, since our hypotheses of lower mediodorsal nucleus and pulvinar volumes and fewer total mediodorsal nucleus neurons in schizophrenic subjects were based on previously published studies
(9–
14).
Discussion
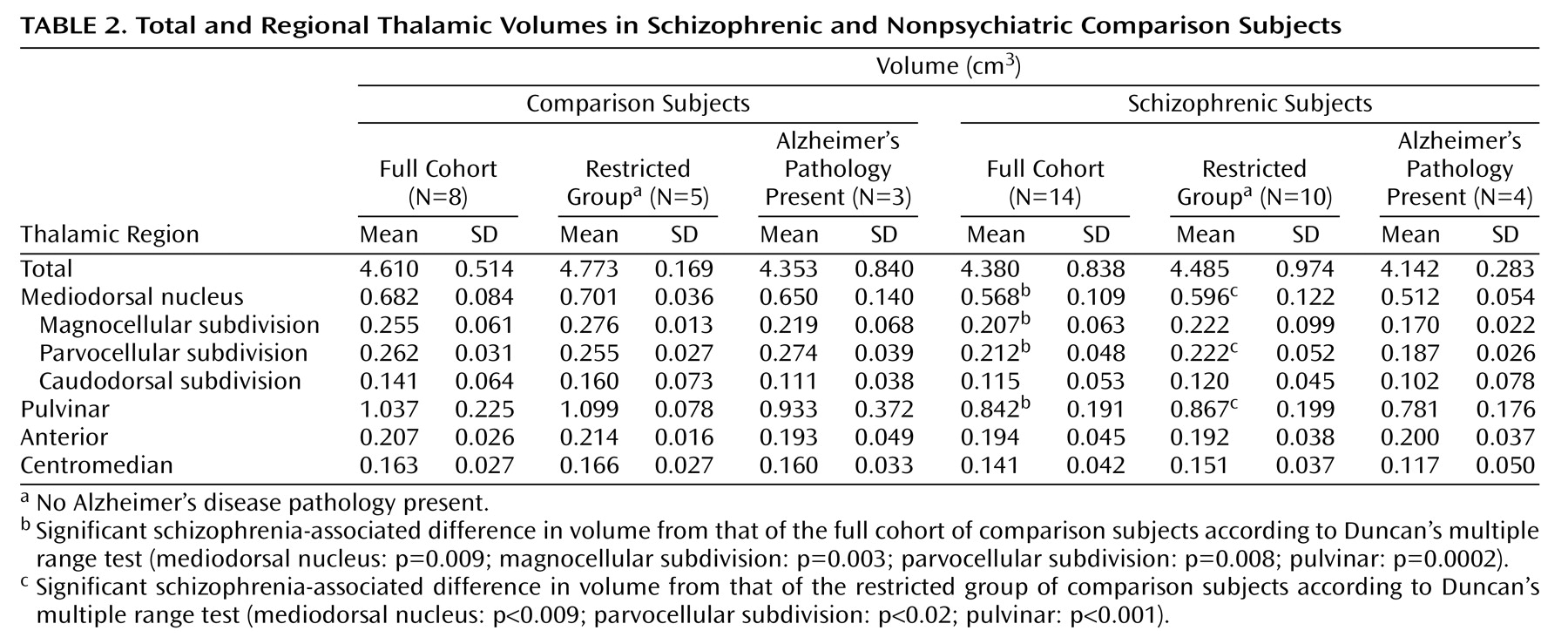
This study corroborates previous reports of schizophrenia-associated volume differences in the mediodorsal nucleus and pulvinar
(9–
14). Since we statistically controlled for variation in brain weight, the observed differences cannot reflect a process that affects the brain globally. Since the volumes of the whole thalamus and of the anterior and centromedian nuclei were not significantly lower, the differences in the mediodorsal nucleus and pulvinar cannot be attributed to a generalized reduction in thalamic size or to the longer average postmortem interval for the specimens from the schizophrenic subjects. Moreover, no correlation emerged between nuclear volumes and either postmortem interval or storage time.
The presence of Alzheimer’s disease in some of the subjects is a potential confounding variable that must be considered. Although the cortical pathology of Alzheimer’s disease might be expected to produce retrograde degenerative changes in the thalamus, resulting in lower volume, the inclusion of comparison specimens with Alzheimer’s disease would be more likely to mask differences between schizophrenic and comparison subjects rather than lead to false positive results. Most important, the differences in mediodorsal nucleus and pulvinar volume and neuron number were statistically significant even when subjects with Alzheimer’s disease were excluded and, therefore, cannot be attributed to Alzheimer’s disease. Finally, our data are virtually identical to recent findings of neuronal loss in the mediodorsal nucleus in a group unaffected by Alzheimer’s disease
(14).
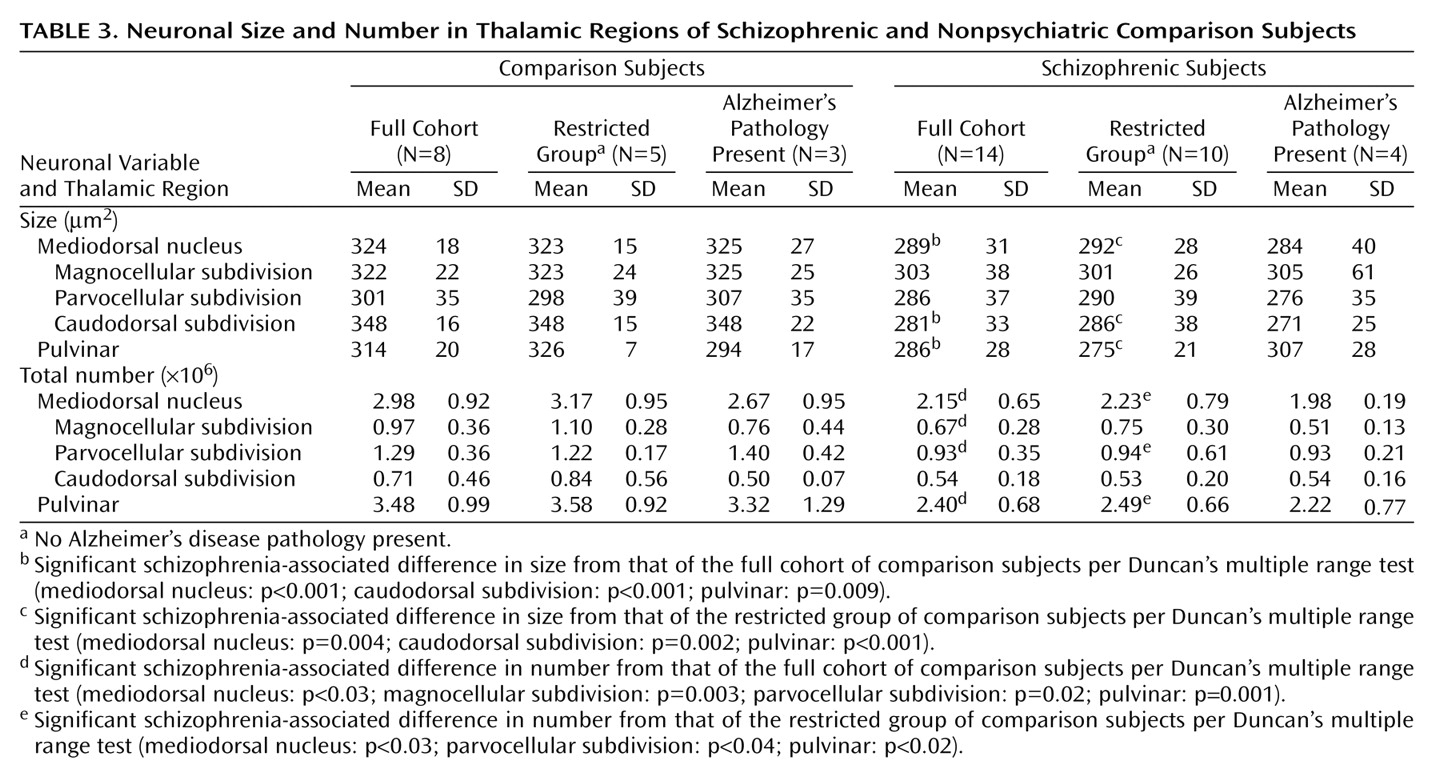
Previous studies have reported 2–4×10
6 total neurons in the normal mediodorsal nucleus with a 27%–45% difference associated with schizophrenia
(10,
13,
14). Variation in total neuronal number may reflect differences in delineating boundaries of the mediodorsal nucleus and its subdivisions, while differences in the percentage of neurons lost in schizophrenic subjects may reflect heterogeneity across different populations. In comparison to previous studies, we found approximately 3.0×10
6 total neurons in the normal mediodorsal nucleus and a 26% difference associated with schizophrenia, in striking agreement with a recent study
(14) that reported 3.5×10
6 neurons in the normal mediodorsal nucleus and a 27% difference associated with schizophrenia. Also in agreement with our findings, that study, which to date is the only other study to our knowledge to have examined subdivisions of the mediodorsal nucleus, found significantly fewer neurons in the parvocellular subdivision. While we found significantly fewer neurons in the magnocellular subdivision as well (only when the full cohort was employed), the previous study’s finding did not achieve statistical significance (p=0.09, two-tailed). The previous study also detected significant neuronal loss in the densocellular division of the mediodorsal nucleus, a division that we did not delineate but which is situated largely in the lateral and dorsal extent of our delineation of the parvocellular subdivision. Conversely, we delineated the caudodorsal subdivision, which was not delineated by the other authors. For the caudodorsal subdivision, mean neuronal counts in the schizophrenic subjects were lower than in the comparison subjects; however, the difference was not statistically significant.
Together, the results of these studies provide further evidence for schizophrenia-associated neuronal loss in the mediodorsal nucleus and suggest this neuronal difference affects the parvocellular subdivision. However, in the absence of larger sample sizes and well-matched specimens without significant neuropathological comorbidities, it would be premature to conclude that other subdivisions are not also affected. Elucidation of differential effects on the subdivisions of the mediodorsal nucleus is complicated by differences that exist among experts regarding the subparcellation mediodorsal nucleus (for examples, see references
3,
14,
19,
24). While our subdivision of the mediodorsal nucleus differed from that of Popken et al.
(14), that difference would not have altered conclusions regarding the nucleus as a whole. It will be important for future work to also address the subdivisions of the pulvinar, since each of its subdivisions also has a unique pattern of efferent and afferent connections. For example, its medial division has projections to the dorsolateral prefrontal cortex similar to those of the mediodorsal nucleus
(29). Thus, schizophrenia-associated pathology in the mediodorsal nucleus and the medial pulvinar may be etiologically linked by virtue of their connections with the dorsolateral prefrontal cortex.
The observed difference in mediodorsal nucleus neuronal size is in agreement with some, but not all, previous reports (see reference
16 for discussion), and a smaller pulvinar neuron size has also been reported in subjects with schizophrenia, but only for Golgi type II neurons
(16). The difference we observed in neuronal size in the mediodorsal nucleus and pulvinar is not likely to reflect a fixation-shrinkage artifact, since tissues were held in fixative long enough to maximize and, thereby, reduce variability in shrinkage. Nor is it likely to be due to the longer postmortem intervals or shorter storage times of the specimens from the schizophrenic subjects, since neuronal size was not correlated with either postmortem interval or storage time. Instead, the size difference may reflect a hypoactive state due to a reduction of the number or activity of excitatory inputs. Within the mediodorsal nucleus, the difference in cell size was most robust in the caudodorsal subdivision, a region that is reciprocally interconnected with the medial surface of the prefrontal cortex
(24). Alternatively, the smaller neuronal size may reflect neurons with less extensive synapses in their efferent fields. The mediodorsal nucleus and pulvinar have extensive reciprocal connections with cortical regions consistently implicated in schizophrenia, including the dorsolateral prefrontal, cingulate, and temporal cortexes
(3,
23,
24,
29); however, the thalamic projections to these regions have not been examined in subjects with schizophrenia.
All of the schizophrenic subjects from whom specimens were taken had received prolonged exposure to antipsychotic medications. A previous postmortem study found that schizophrenia-associated volume differences in the mediodorsal nucleus were independent of antipsychotic exposure
(11). However, an influence of antipsychotic medication cannot be excluded. Although schizophrenic subjects are at greater risk for alcohol and other substance abuse
(30), none of the subjects in this study had such histories. Thus, group differences in substance abuse cannot account for the present observations.
In summary, our findings provide further evidence in support of schizophrenia-associated abnormalities in the mediodorsal nucleus and pulvinar, the major association nuclei of the thalamus, both of which have extensive projections to other brain regions implicated in schizophrenia. As we have discussed more fully elsewhere
(9), schizophrenia-associated changes in thalamic nuclei may be etiologically related to changes in their cortical fields. It is not clear, however, whether the thalamic changes arise earlier in development and give rise to the cortical changes or whether changes in the cortex generate the thalamic abnormalities. Thalamic nuclei in addition to those that have already been studied need to be assessed systematically to test the specificity of the observed changes to the mediodorsal nucleus and pulvinar. The phenotype of the affected neurons in the mediodorsal nucleus and pulvinar also remains to be determined. Determining whether thalamic cell loss involves projection neurons as opposed to interneurons may have bearing on the relationship between pathology in the thalamus and its cortical fields. While changes in thalamic projection neurons might induce changes in their cortical targets, changes in thalamic interneurons could be secondary to alterations in afferent projections to the thalamus.