Suicide is a major public health concern. Although there have been several studies that have suggested that suicide is associated with neurobiological abnormalities, the precise molecular mechanisms associated with suicidal behavior remain unclear. With this respect, several lines of evidence suggest the involvement of neurotransmitter receptors (e.g., α
2-adrenergic, β-adrenergic, and the serotonin [5-HT] receptors 5-HT
1A, 5-HT
2A, and 5-HT
2C) in the pathophysiology of suicide. Greater agonist binding to α
2-adrenergic receptors and a higher number of binding sites for β-adrenergic, 5-HT
2A, and 5-HT
2C receptors have been shown in the postmortem brain of suicide victims, while fewer 5-HT
1A binding sites have also been reported (reviewed by Gross-Iseroff et al.
[1]).
Previous studies have suggested that apart from the neurotransmitter receptors, the signaling cascade through which these receptors mediate their physiological responses may be involved in the pathophysiology of suicide. Receptors such as 5-HT
2A and 5-HT
2C are linked with the phosphoinositide signaling system, whereas 5-HT
1A, α
2-adrenergic, and β-adrenergic receptors are linked with the adenylyl cyclase–cAMP signaling system. In the phosphoinositide signaling system, our previous studies have shown less [
3H]phorbol dibutyrate binding to protein kinase C
(2) and lower catalytic activity of phosphoinositide-specific phospholipase C
(3) in the prefrontal cortex of suicide victims. In addition, Pacheco and Jope
(4) and Jope et al.
(5) have reported an alteration in the overall phosphoinositide signaling system in the postmortem brain of suicide victims. These studies thus suggest an abnormality in the phosphoinositide signaling system in the postmortem brain of suicide victims. Contrary to this, the involvement of the adenylyl cyclase–cAMP signaling system in the pathophysiology of suicide has not been evaluated in greater detail. In this signaling system, receptor-mediated activation of G
sα or G
iα proteins causes modulation of adenylyl cyclase, which in turn causes the conversion of ATP to cAMP. cAMP serves as a second messenger and activates the protein kinase A enzyme. Protein kinase A then phosphorylates various substrate proteins in cells, thereby mediating a variety of hormonal and physiological responses, including receptor down-regulation, desensitization, altered neurotransmitter release, and activation or repression of gene expression
(6,
7). In the postmortem brain of suicide victims, higher levels of G
s and lower levels of G
i have been shown
(8,
9), and lower levels of GTPγS-stimulated and forskolin-stimulated cAMP formation have been reported
(10). Since protein phosphorylation mediated by protein kinase A is the major mechanism of signal transduction in this signaling pathway, studying the status of protein kinase A beyond the generation of cAMP provides direct evidence of abnormal cellular signaling at the level of functional response.
Protein kinase A exists as a tetramer holoenzyme that consists of two regulatory and two catalytic subunits
(11,
12). The regulatory subunits regulate the activity of the catalytic subunits and have been recognized as the proteins that bind cAMP. In the holoenzyme state, protein kinase A exists in an inactive form. Following an increase in intracellular cAMP, the regulatory subunits bind to cAMP, which results in the dissociation of the holoenzyme into a dimeric regulatory and two monomers of catalytic subunits
(13). The free catalytic subunits can then phosphorylate substrates or translocate into the nucleus by passive diffusion and phosphorylate nuclear substrates
(14). Thus, both catalytic and regulatory subunits are important in facilitating protein kinase A-mediated functions.
In the present investigation, we evaluated the role of protein kinase A in suicidal behavior by examining both its regulatory and catalytic properties in the prefrontal cortex (Brodmann’s area 9) of suicide victims and nonpsychiatric comparison subjects. We elected to analyze prefrontal area 9 because of our long-standing interest in the dorsolateral prefrontal cortex. Previously, we have observed a higher number of binding sites for 5-HT
2A receptors and abnormalities in the phosphoinositide signaling system in this brain area of suicide victims
(2,
3,
15). Very recently, we have shown lower activation and expression of another phosphorylating enzyme, extracellular-regulated kinases, in this brain area of suicide victims
(16). Besides these findings, this brain area has been shown to play a relevant role in mood regulation
(17) and has been implicated in the pathophysiology of suicide and affective disorders in a number of other neurochemical studies (reviewed in reference
18).
Results
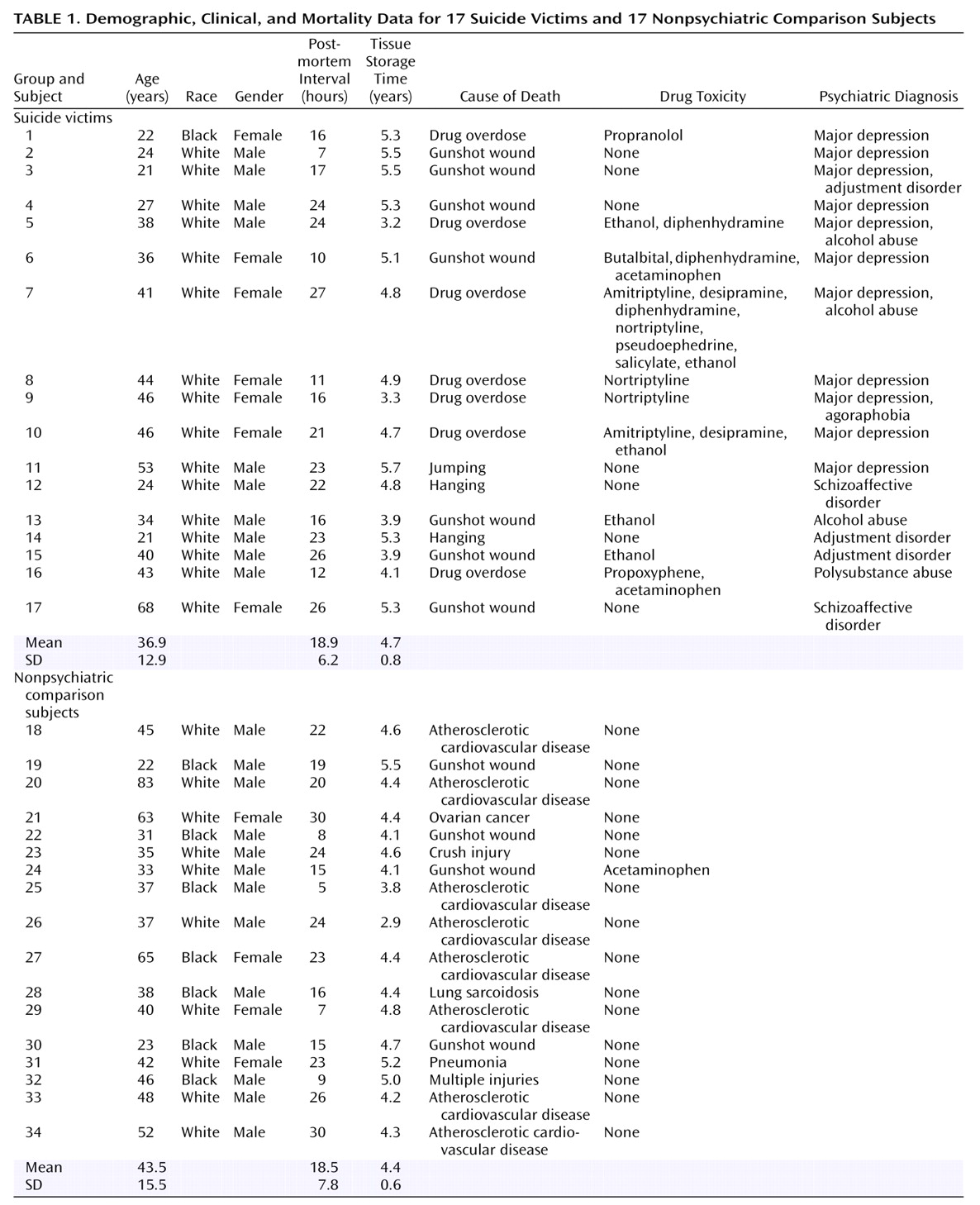
Detailed clinical and demographic characteristics of the suicide victims and the nonpsychiatric comparison subjects are shown in
Table 1. There were no significant differences in postmortem interval (t=–0.12, df=32, p=0.90) or age (t=1.34, df=32, p=0.19) between the two groups.
[3H]cAMP Binding to Protein Kinase A
The maximum number of binding sites (B
max) and the apparent dissociation constant (K
D) in both the membrane and cytosol fractions were determined by using different concentrations of [
3H]cAMP (0.25–10 nM). Nonspecific binding was determined in the presence of 5 μM of cAMP.
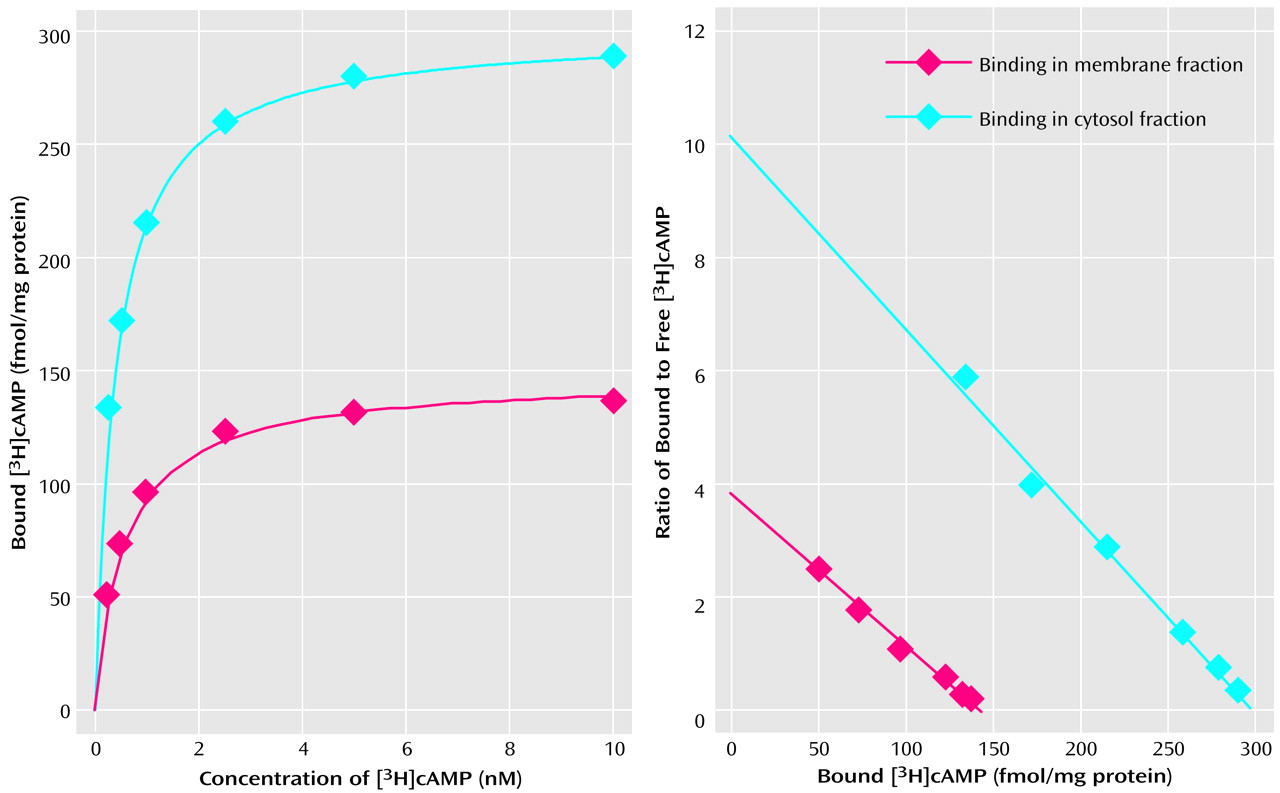
Figure 1 represents a typical saturation isotherm and a Scatchard plot of [
3H]cAMP binding to the membrane and cytosol fractions of the prefrontal cortex of a nonpsychiatric comparison subject. It was observed that specific binding was saturable and exhibited a single class of binding site. Nonspecific binding was nonsaturable and linear with concentrations of 0.25–10 of nM [
3H]cAMP. It was observed that B
max of [
3H]cAMP binding to protein kinase A was greater in the cytosol than in the membrane fraction.
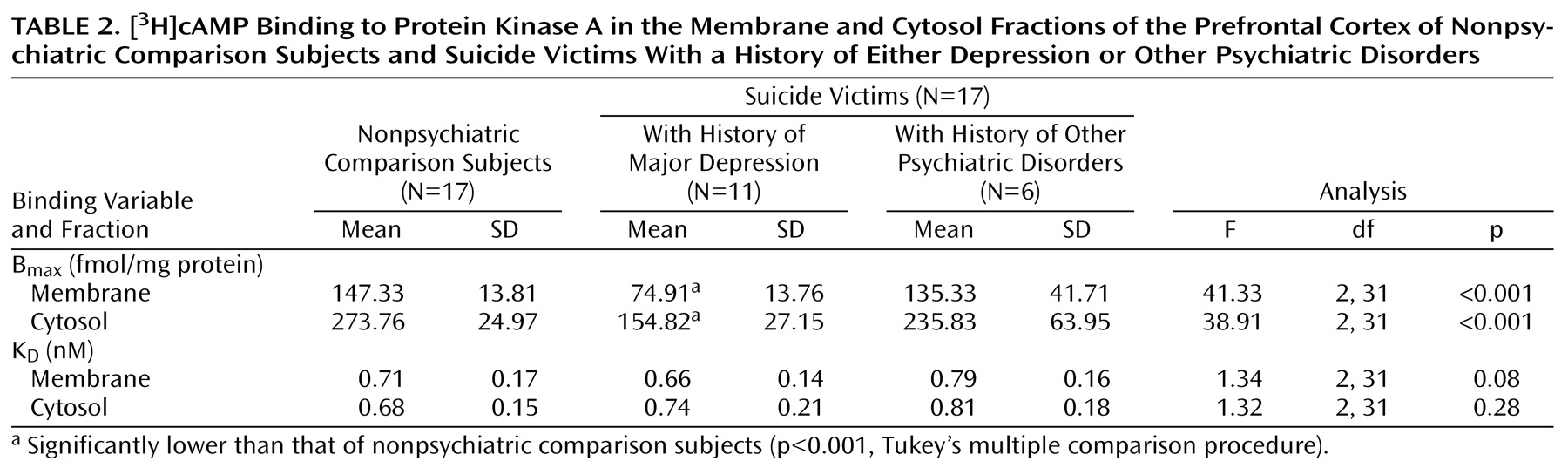
A comparison of B
max and K
D values of [
3H]cAMP binding in the membrane and cytosol fractions of the prefrontal cortex between suicide victims and nonpsychiatric comparison subjects is given in
Table 2. B
max of [
3H]cAMP binding to protein kinase A was significantly smaller in both the membrane (t=5.05, df=19.9, p<0.001) and cytosol (t=6.00, df=20.60, p<0.001) fractions of the prefrontal cortex of suicide victims than in the nonpsychiatric comparison subjects, whereas there were no significant between-group differences in K
D values in either the membrane (t=0.04, df=32, p=0.97) or the cytosol (t=–1.42, df=32, p=0.17) fractions.
We then examined whether these effects were related to age, postmortem interval, or gender. However, no significant correlations were observed between Bmax of [3H]cAMP binding to protein kinase A and age (membrane: r=–0.06, p=0.74; cytosol: r=0.04, p=0.82), postmortem interval (membrane: r=–0.08, p=0.67; cytosol: r=–0.04, p=0.83), or gender (membrane: r=0.21, p=0.23; cytosol: r=0.26, p=0.12) (df=32 for all analyses).
We further examined if the lower B
max of [
3H]cAMP binding was generalized across all the suicide victims or was related to presence of a mental disorder. For this purpose, we divided the suicide victims into those who were diagnosed with major depression and those with other psychiatric disorders. Out of 17 suicide victims, 11 had a history of major depression. We observed that B
max of [
3H]cAMP binding to protein kinase A was significantly lower only in those suicide victims who had a previous history of major depression. It is of interest that B
max of [
3H]cAMP binding in the suicide victims with a history of other psychiatric disorders was only slightly lower (15%–20%) than that of the nonpsychiatric comparison subjects and was not significantly different (
Table 2).
To rule out the possibility that the lower amount of [3H]cAMP binding was related to drug toxicology, we compared the membrane and cytosol Bmax values and found them to be similar in depressed suicide victims with drug toxicology (membrane: mean=76.85 fmol/min/mg protein [SD=16.65]; cytosol: mean=161.14 fmol/min/mg protein [SD=30.59]) and in those without drug toxicology (membrane: mean=71.50 fmol/min/mg protein [SD=7.18]; cytosol: mean=143.75 fmol/min/mg protein [SD=18.13]).
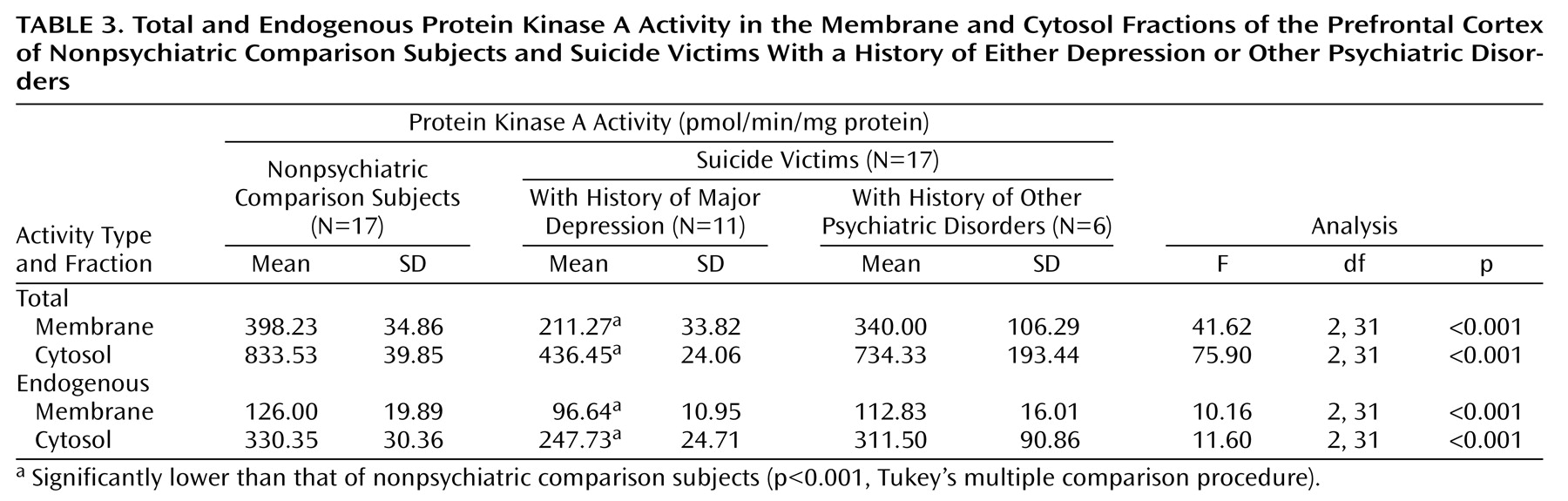
Protein Kinase A Activity
Total and endogenous protein kinase A activity in the prefrontal cortex of suicide victims and nonpsychiatric comparison subjects are presented in
Table 3. We observed significantly less total and endogenous protein kinase A activity in the membrane fraction (total: t=6.0, df=20.6, p<0.001; endogenous: t=3.93, df=32, p<0.001) and cytosol fraction (total: t=6.42, df=17.5, p<0.001; endogenous: t=3.55, df=23.1, p=0.002) of the prefrontal cortex of suicide victims relative to that of nonpsychiatric comparison subjects. These changes in protein kinase A activity were much greater, however, in the presence of cAMP (35%–55%) than in the absence of cAMP (approximately 20%). Further, we did not observe in either the membrane or cytosol fractions any significant effects of age (total activity: r=0.21, p=0.23, and r=0.17, p=0.33, respectively; endogenous activity: r=–0.05, p=0.79, and r=0.11, p=0.53), postmortem interval (total activity: r=–0.06, p=0.72, and r=0.02, p=0.92; endogenous activity: r=–0.12, p=0.48, and r=–0.09, p=0.59), or gender (total activity: r=0.27, p=0.11, and r=0.30, p=0.08; endogenous activity: r=0.20, p=0.23, and r=0.25, p=0.15) (df=32 for all analyses).
Since we observed a lower B
max of [
3H]cAMP binding only in suicide victims with major depression, we sought to determine if protein kinase A activity followed a similar pattern of changes. As with B
max of [
3H]cAMP binding, both total and endogenous protein kinase A activity were significantly lower only in those suicide victims who had a previous history of major depression (
Table 3). However, those suicide victims who were diagnosed with other psychiatric disorders had a slight but nonsignificant decrease (approximately 12%–20%) in both total and endogenous protein kinase A activity (
Table 3).
In an analysis of drug toxicology effects, similar total and endogenous protein kinase A activity was seen in depressed suicide victims with drug toxicology (total activity: membrane mean=211.71 pmol/min/mg protein [SD=42.55], cytosol mean=440.71 pmol/min/mg protein [SD=16.41]; endogenous activity: membrane mean=96.85 pmol/min/mg protein [SD=11.50], cytosol mean=236.71 pmol/min/mg protein [SD=24.49]) and depressed suicide victims without drug toxicology (total activity: membrane mean=210.50 pmol/min/mg protein [SD=13.79], cytosol mean= 429.00 pmol/min/mg protein [SD=35.95]; endogenous activity: membrane mean=96.25 pmol/min/mg protein [SD=11.58], cytosol mean=267.00 pmol/min/mg protein [SD=7.48]).
Discussion
The present investigation reveals several interesting findings. For instance, the amount of [
3H]cAMP binding was significantly lower in the membrane and cytosol fractions of the prefrontal cortex (Brodmann’s area 9) of suicide victims without any change in affinity. This lower binding was accompanied by lower total (in presence of cAMP) and endogenous (in absence of cAMP) protein kinase A activity. The lower level of protein kinase A activity was more pronounced in the presence of cAMP than in the absence of cAMP. We observed that the lower [
3H]cAMP binding and protein kinase A activity was restricted to those suicide victims with a previous history of major depression rather than those with a history of other psychiatric disorders. These changes were not related to antidepressant toxicology or other confounding variables such as postmortem interval, age, or gender. Our study thus indicates that lower [
3H]cAMP binding and protein kinase A activity is related to depression and that confounding variables—including antidepressant treatment—do not affect these measures. However, our study has some limitations. The numbers of subjects in each subgroup of suicide victims with other psychiatric disorders, as well as in the depressed group who showed positive antidepressant toxicology, were small. Therefore, it is premature to state that alterations in protein kinase A are specific to depression. It is interesting to mention that Shelton et al.
(24) and Manier et al.
(25) have found lower β-adrenergic receptor-stimulated protein kinase A activity in the fibroblasts of depressed patients, suggesting that depression may be associated with abnormalities in protein kinase A.
Although its status in depression remains unclear, protein kinase A has been studied extensively in affective disorders and in the mechanism of action of antidepressants. For example, Rahman et al.
(26) reported lower [
3H]cAMP binding in the cytosol fraction of various postmortem brain areas of subjects with bipolar affective disorder. In contrast, Fields et al.
(27) reported higher protein kinase A activity in the temporal cortex of subjects with bipolar affective disorder. On the other hand, preclinical studies suggest that chronic treatment with imipramine, tranylcypromine, or ECT causes translocation of protein kinase A in the rat brain, resulting in greater protein kinase A activity in the particulate and less protein kinase A activity in the cytosol fraction
(28). These effects are produced selectively by antidepressants and are not found with other psychotropic drugs. In addition, 5-HT and norepinephrine reuptake inhibitors increase the binding of cAMP to the regulatory subunit of protein kinase A
(29–
31). With regard to the CNS role of protein kinase A in depression, so far there is only one study to our knowledge that has examined protein kinase A in the postmortem brain of depressed suicide victims. In this study, Lowther et al.
(32) found no significant change in [
3H]cAMP binding. In contrast, our results indicate that not only [
3H]cAMP binding to the regulatory subunit of protein kinase A but also total and endogenous protein kinase A activity are lower in the postmortem brain of suicide victims. The reason for this discrepancy is not clear but may tentatively be attributed to the brain areas studied. Whereas we studied Brodmann’s area 9, Lowther et al.
(32) studied Brodmann’s areas 7, 10, and 21/22. Since we did not perform these studies in other brain areas, it is difficult to speculate whether these changes are restricted to Brodmann’s area 9 or are also present in other brain areas. An interesting observation by Lowther et al.
(32) was that [
3H]cAMP binding was significantly lower in those suicide victims who were treated with antidepressants. In our study, although the number of depressed suicide victims who showed positive drug toxicology was small, nonetheless the B
max of [
3H]cAMP binding and protein kinase A activity were similar among those depressed suicide victims with antidepressant toxicology present and those with no antidepressant toxicology.
Two other interesting observations were made in the present study: 1) the differences in [
3H]cAMP binding and protein kinase A activity were present in both the membrane and cytosol fractions, and 2) protein kinase A activity was lower both in the presence and in the absence of cAMP. Upon activation by cAMP, protein kinase A translocates from the cytosol to the particulate fraction. It has been reported that the translocation of protein kinase A occurs through different compartments, with consequent phosphorylation of selective substrates
(31). In our study, we did not find any translocation of protein kinase A, since lower [
3H]cAMP binding as well as lower protein kinase A activity were seen in both the membrane and cytosol fractions. This suggests that abnormalities in cAMP-mediated functions are not localized to a certain compartment but rather are generalized. We presume that the observed lower [
3H]cAMP binding occurred because of less abundance of regulatory subunits of protein kinase A available to bind the cAMP. Similarly, there are less catalytic subunits available; therefore less protein kinase A activity occurs even in the absence of cAMP. In this context, it is interesting to mention that lower [
3H]cAMP binding
(26) and greater protein kinase A activity
(27) have been reported in the postmortem brain of subjects with bipolar affective disorder. These investigators have speculated that this opposite effect may occur because of the greater relative abundance of free catalytic subunits to regulatory subunits.
The mechanism of lower [
3H]cAMP binding and protein kinase A activity in the prefrontal cortex of suicide victims is not clear at the present time. However, as we have mentioned, there is a strong possibility that there may be less expression of catalytic and regulatory subunits of protein kinase A, which may be related to the abnormalities upstream in the adenylyl cyclase–cAMP signaling system. A higher number of β-adrenergic and α
2-adrenergic receptors
(1), along with greater expression of G
sα and fewer G
iα proteins
(8,
9), have been shown in the postmortem brain of suicide victims, suggesting greater G
s- and G
iα-mediated functions in suicidal behavior. In view of these observations, it is quite possible that an increase in cAMP levels due to higher levels of G
sα or lower levels of G
iα protein may cause adaptive changes in the regulatory or the catalytic subunits, thereby reducing [
3H]cAMP binding to protein kinase A and the enzyme’s activity as a compensatory event. In support of this notion, it has been reported that a sustained elevation in the intracellular levels of cAMP causes adaptive changes in both catalytic and regulatory subunits of protein kinase A
(11,
12).
The relevance of impaired protein kinase A to the pathophysiology of depression/suicide remains to be elucidated; however, a decrease in the catalytic and regulatory properties of protein kinase A subunits may have important functional consequences, since it has been established that many biological responses are regulated by the state of the phosphorylation of specific substrate proteins, which in turn are involved in the regulation of cellular functions, such as neurotransmitter release
(33), receptor desensitization
(34), and gene expression
(35,
36). Thus, a decrease in protein kinase A may result in significant impairments in various physiological functions in depression and suicidal behavior. At this juncture, it is difficult to relate our findings to Brodmann’s area 9, where we observed changes in protein kinase A, since the levels of cAMP differ from one brain area to other and even from cell to cell. Nonetheless, several neurochemical abnormalities have been reported in Brodmann’s area 9 of suicide victims, including abnormalities in serotonergic and adrenergic receptors
(1). We and other investigators have also reported alterations in components of the phosphoinositide signaling system, such as protein kinase C
(2), phospholipase C
(3), and G proteins
(9) in Brodmann’s area 9 of suicide victims. In most signaling pathways, protein phosphorylation represents a crossroad where extensive cross-talk occurs between various signaling systems
(37). Given the alterations in multiple signaling system, it is quite possible that Brodmann’s area 9 may have physiological significance in suicidal behavior.
In conclusion, our observations of lower protein kinase A activity and [3H]cAMP binding in the prefrontal cortex of suicide victims indicate that abnormalities in the adenylyl cyclase–cAMP signaling system may be associated with the pathophysiology of suicide. Additional studies are required to delineate the physiological significance of these findings and whether these differences are mediated through receptors or are independently abnormal.