Stressful life events are frequently associated with the onset of episodes of major depression
(1,
2). Elevated levels of plasma cortisol
(3,
4) and deficits in declarative memory mediated by the hippocampus
(5–
7) have also been reported in subgroups of patients with unipolar major depressive disorder. Preclinical studies have confirmed that chronic psychosocial stress and cortisol administration inhibit neurogenesis in the dentate gyrus
(8–
10) and cause atrophy or remodeling of the apical dendrites of the pyramidal neurons in the CA3 region of the hippocampus (reviewed in references
11–13).
Morphometric techniques based on magnetic resonance imaging have been used to determine whether these preclinical findings of hippocampal damage can be extended to patients with major depression and other stress-related disorders. Over the last 5 years, several groups have confirmed smaller than normal volumes of the left hippocampus
(14,
15) and of the left and right hippocampus
(5,
16,
17) in unipolar major depressive disorder. A smaller hippocampal volume was correlated with the total duration of depression
(5,
16). Other groups, however, have not found significant differences in hippocampal volume between patients with unipolar major depressive disorder and healthy subjects
(18–
23). Reports on hippocampal volume in bipolar disorder are also mixed, with reports of larger
(24) and smaller
(25) hippocampal volumes, as well as no difference in volume between bipolar disorder patients and normal subjects
(26).
In addition to methodological issues such as slice thickness and definition of hippocampal landmarks, several clinical and biological factors may help explain these disparate findings in hippocampal morphometry in major depressive disorder. A parallel corpus of studies in adults with a history of childhood abuse has confirmed the presence of smaller hippocampal volumes using similar morphometric magnetic resonance imaging (MRI) techniques. Patients with posttraumatic stress disorder (PTSD) associated with severe and repeated physical and/or sexual abuse in childhood have been shown to have significantly smaller left hippocampal volumes
(27,
28). Adult women with a history of childhood abuse have smaller left hippocampal volumes despite different psychiatric diagnoses
(28). Women with borderline personality disorder and a history of childhood trauma also have been reported to have smaller hippocampal volumes, compared to healthy women
(29).
According to the most recent data from the National Child Abuse and Neglect Data System, an estimated 826,000 cases of child maltreatment were reported in the United States in 1999
(30). Half of these cases were instances of neglect, 21% were instances of physical abuse, and 11% were instances of sexual abuse. Comorbid major depressive disorder occurs in approximately 40%–50% of patients with PTSD
(31,
32). Despite preclinical and clinical evidence suggesting that severe stress early in life is associated with persistent hypothalamic-pituitary-adrenal (HPA) axis abnormalities and a reduction of hippocampal volume in adulthood, morphometric studies in major depressive disorder have neither reported nor controlled for a history of early childhood trauma. The inconsistent findings on hippocampal volume in major depressive disorder therefore may be partly due to the uncontrolled and variable inclusion of women with a history of childhood trauma.
The present study measured the volumes of the whole hippocampus and of control brain regions in depressed subjects and healthy comparison subjects for whom a detailed evaluation of childhood history of physical and/or sexual abuse was obtained. In view of the aforementioned preclinical and clinical literature showing persistent brain changes after adverse experiences early in life, we hypothesized that depressed patients with a history of early childhood abuse would have smaller hippocampal volumes, compared to depressed subjects and healthy subjects without a history of childhood trauma.
Method
Subjects
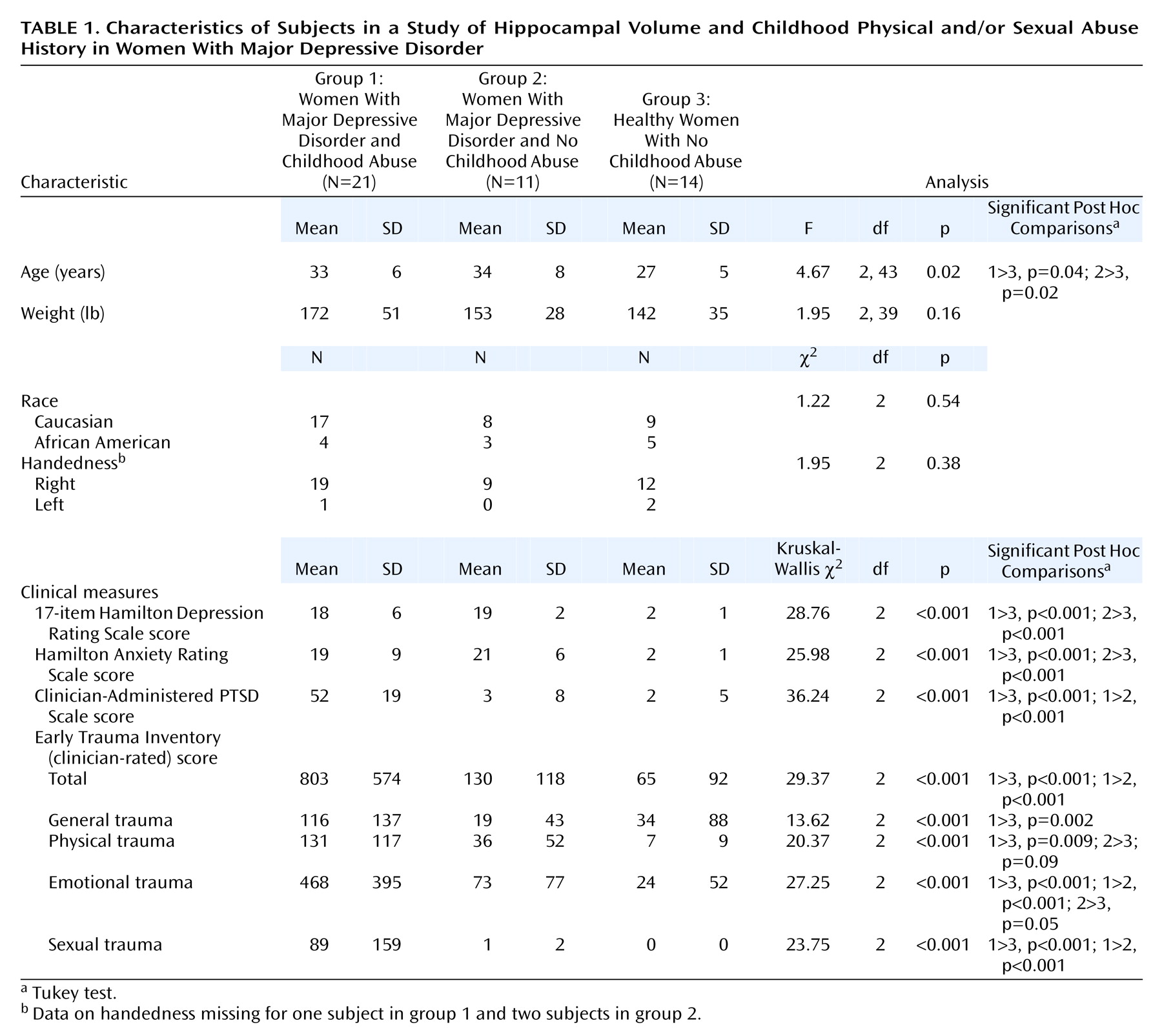
Forty-six women were recruited through newspaper advertisements and flyers and were admitted as inpatients to the Emory University Hospital General Clinical Research Center. The protocol was approved by both the Emory University Institutional Review Board (the Human Investigators Committee) and the General Clinical Research Center Human Subjects Committee. All subjects provided written informed consent before participation. Twenty-one subjects who fulfilled criteria for childhood physical and/or sexual abuse and major depressive disorder, 11 patients who fulfilled criteria for current major depressive disorder but had not experienced childhood abuse, and 14 healthy subjects without early trauma history were included in the study. All subjects received monetary compensation for participation.
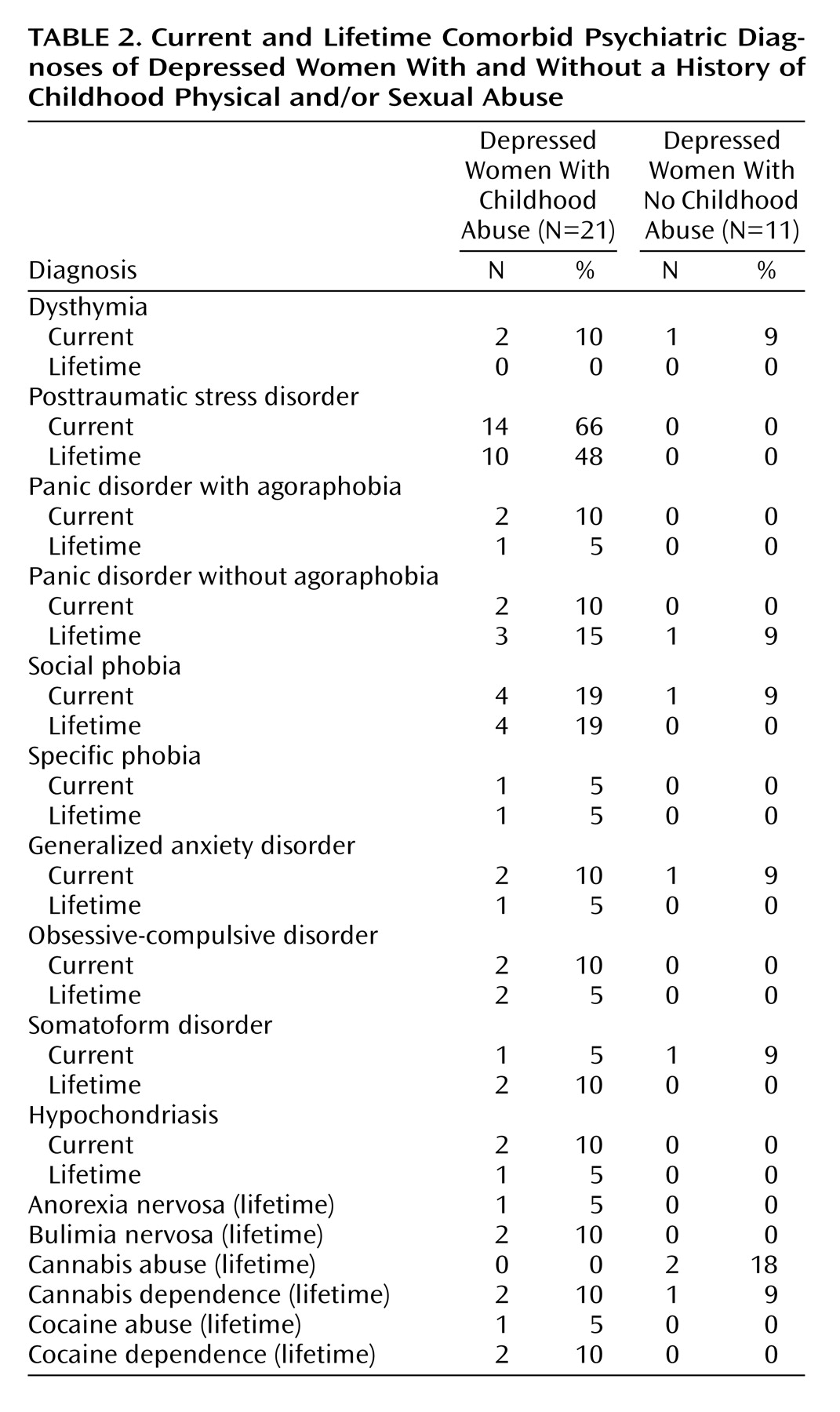
Depressed patients were included if they fulfilled criteria for major depressive disorder on the basis of the Structured Clinical Interview for DSM-IV Axis I Disorders
(33). Severity of depression was measured with the 17-item Hamilton Depression Rating Scale
(34) and the Zung Depression Scale
(35).
The clinician-rated Early Trauma Inventory
(36) was administered to determine the presence of early life trauma and to evaluate the extent of the trauma. The Early Trauma Inventory assesses the number, frequency, duration, and subjective impact of different types of traumatic experiences (physical, sexual, and emotional abuse and general traumas) and has been shown to be a valid and reliable measure of early trauma. Considerable effort was made to obtain independent validation of abuse from court, social service, and/or medical records and from family members or friends. Independent verification was possible for 11 (52%) of 21 subjects. The Clinician-Administered PTSD Scale
(37) was also administered to confirm the presence of PTSD symptoms and evaluate their severity.
Since traumatic experiences early in childhood (and not peripubertal/postpubertal trauma) have been consistently associated with persistent HPA axis and hippocampal structural abnormalities and an increased risk for adulthood disorders
(38), we restricted this study to women with a history of prepubertal physical and/or sexual abuse. Stringent criteria for physical and/or sexual abuse were used to reduce variability related to differences in types of trauma. Depressed women with a history of childhood abuse were included if the abuse occurred before the subject’s first menstrual period at a frequency of at least once a month for more than a year. Sexual abuse was defined as having been forced to touch another person’s intimate parts, having been touched in intimate parts, or attempted or completed vaginal, oral, or anal intercourse. Physical abuse was defined as having been spanked, hit, kicked, or choked in a way that left bruises or injuries; having been attacked with a weapon; or having been tied up or locked in a room or a closet
(39). Women with major depressive disorder and healthy women without a history of childhood physical and/or sexual abuse, parental loss, or exposure to other traumatic events formed the comparison group.
The healthy women had no current or past history of psychiatric illness, including alcohol or other substance abuse or dependence. Subjects were excluded from the study if they had a major medical illness, significant head trauma, irregular menses, or any other axis I disorder, including bipolar disorder, schizophrenia, and schizoaffective disorder. Those with a contraindication for an MRI, including having a cardiac pacemaker, were excluded.
The subjects were free of hormonal (except for oral contraceptives) or psychotropic medication at the time of the study. The subjects were part of a larger project on the neurobiology of early trauma
(39,
40).
MRI
MRI acquisition
Subjects were imaged with a 1.5-T General Electric Signa device (General Electric, Milwaukee) by using a tilted coronal three-dimensional volume spoiled gradient recoil sequence with TR=25 msec, TE=5 msec, NEX (number of excitations)=2, matrix 256×256, and field of view=22 cm. This resulted in 124 axial 1.5-mm contiguous slices.
MRI processing
Images were transferred through the computer network to a Sun Sparc Ultra 80 workstation (Sun Microsystems, Santa Clara, Calif.). All images were resliced from axial images into 1-mm isometric voxel coronal images that were perpendicular to the long axis of the hippocampus. The boundaries of the hippocampus were traced manually with a mouse-driven cursor by using the ANALYZE program (Mayo Foundation, Rochester, Minn.).
Measurement of hippocampal volume
Anatomic guidelines for the hippocampus were based on the work of Watson et al.
(41) and Duvernoy
(42) and were modified in consultation with a neuroradiologist who is an expert in hippocampal anatomy
(43–
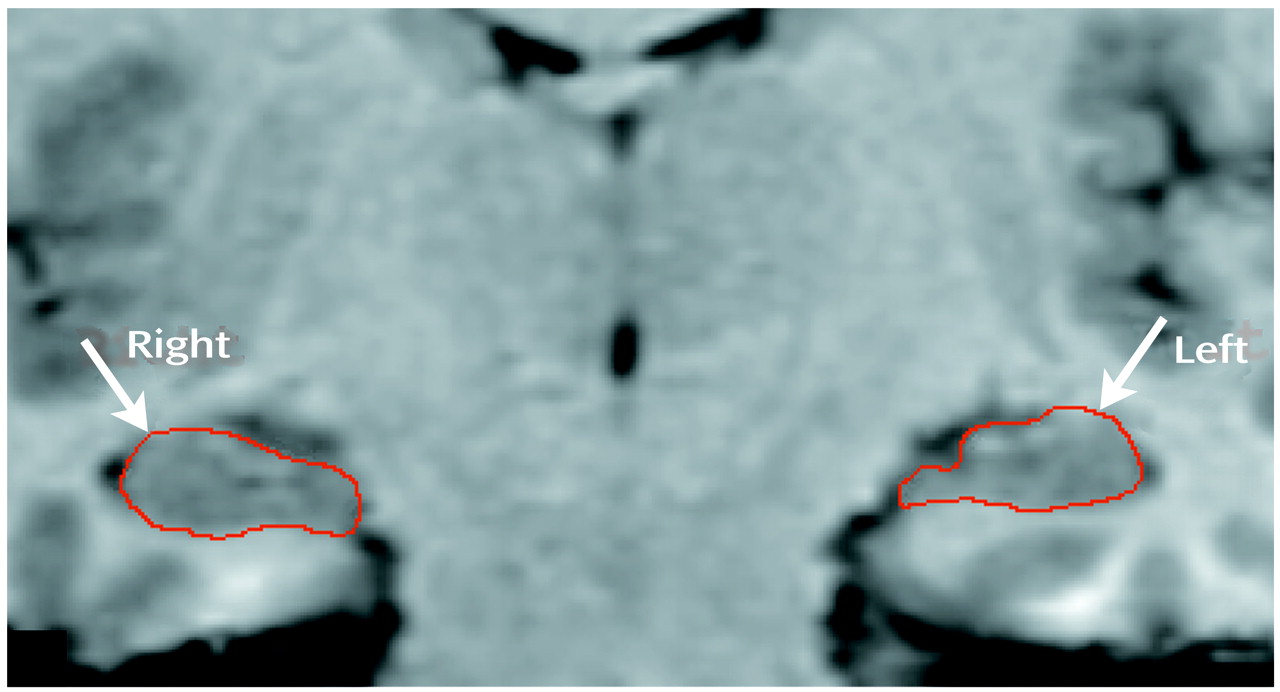
46). After extensive training in hippocampal anatomy, a single rater who was blind to the subjects’ diagnoses (M.V.) traced the hippocampal boundaries of the patients and the healthy comparison subjects (
Figure 1). Structures included in the hippocampal volume were the gray matter of the hippocampus proper, dentate gyrus, subicular complex, alveus, and fimbria. The parahippocampal gyrus, tail of the caudate, fornix, amygdala, and CSF around the hippocampus were excluded. Posteriorly, the start slice was defined as the slice 3 mm anterior to where the crura of the fornix separate from the hippocampus. The CSF of the temporal horn of the lateral ventricle and white matter tracts identified the lateral and inferior boundaries, respectively. Anteriorly, the hippocampus was reliably differentiated from the amygdala by using the criteria of Watson et al.
(41). The CSF in the uncal recess of the temporal horn, when visible, was the most reliable boundary between the hippocampal head and the amygdala. In instances where the uncal recess was not visible, the alveus was used as the boundary to separate the hippocampus and amygdala. If neither the uncal recess nor the alveus was obvious, a straight line was drawn connecting the plane of the inferior horn of the lateral ventricle with the surface of the uncus. The average number of slices traced for each hippocampus was 24 (SD=2).
The hippocampus was segmented into the head, body, and tail to evaluate regional differences in hippocampal volume between the groups and to allow comparison of the results of this study with those of previous studies that measured only the hippocampal body
(14,
47). The body (mid-hippocampal segment) included 10 15-mm coronal slices between the superior colliculus and the bifurcation of the basilar artery, with the first slice anterior to superior colliculus
(27,
45,
47). The tail was defined as all slices posterior to the end slice of the body.
The region-of-interest module of the ANALYZE software package calculated volumes for each slice based on the cross-sectional area measure multiplied by the slice thickness. The final volumes of the right and left whole, head, body, and tail of the hippocampus were calculated by summing the volumes of the individual slices for each region.
Measurement of control brain regions
The temporal lobe of subjects was measured by using previously described methods
(48). The whole brain volume was assessed by using the auto-trace mode in the ANALYZE program and included the gray matter, white matter, and CSF of both cerebral hemispheres, the cerebellum, and the brainstem above the level of the pons.
Interrater reliability
Two raters traced the hippocampus in a subgroup of 12 randomly selected subjects, and the interrater correlation coefficients were calculated. Interrater reliability was determined with the intraclass correlation coefficient (ICC) and one-way analysis of variance (ANOVA) for volumetric assessments of the hippocampus by two raters. The ICCs were 0.9 for the left hippocampus and 0.8 for the right hippocampus.
Statistical Analysis
Group differences between continuous variables such as age and weight were analyzed by using ANOVA. Categorical variables, including race, handedness, and presence or absence of PTSD and alcohol and other substance abuse or dependence, were evaluated by using chi-square tests. Psychometric scale score differences among the three groups were evaluated by using the Kruskal-Wallis nonparametric multiple comparisons test. Comparisons of volumetric measures were conducted by using multivariate analysis of covariance, with whole brain volume, age, race, education, presence of PTSD, and presence of alcohol and other substance abuse or dependence as covariates. Spearman’s rho correlations were used to analyze the relationship between hippocampal volume and the clinical variables.
Discussion
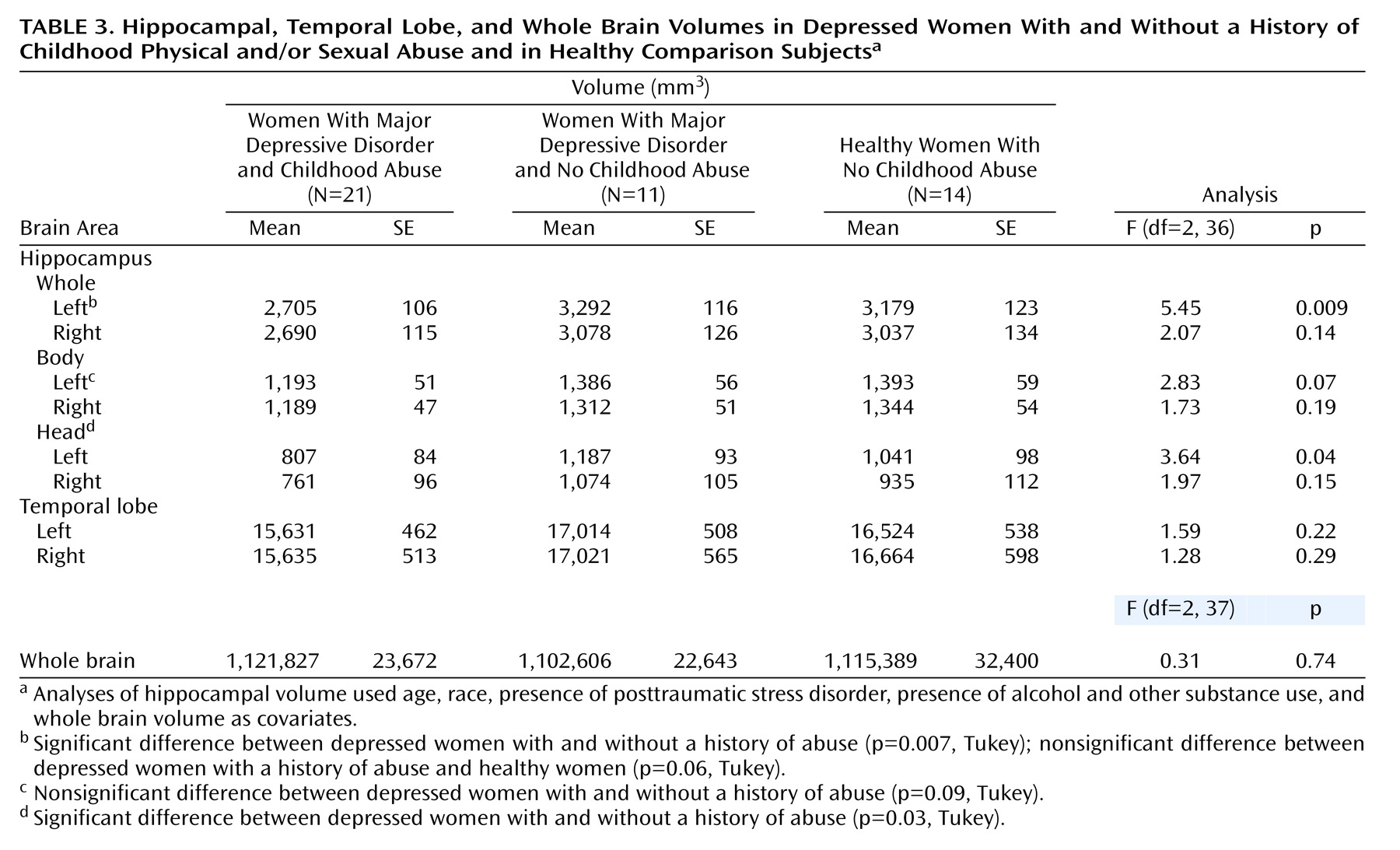
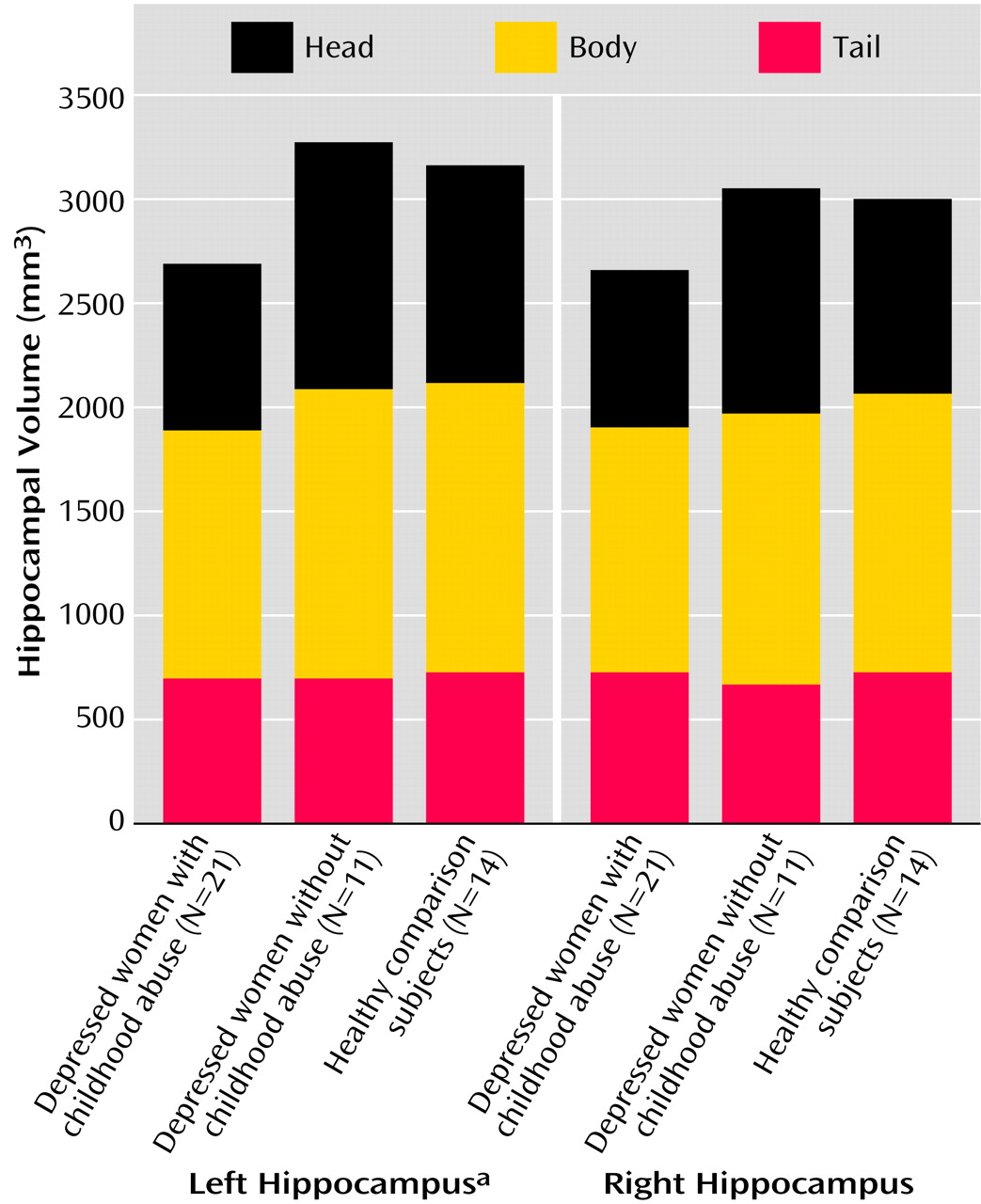
This study demonstrates that adult women with major depressive disorder and a history of severe and repeated physical and/or sexual abuse in childhood had smaller left hippocampal volumes, compared to depressed women without a history of abuse and compared to healthy subjects. Hippocampal volumes in depressed women without a history of childhood abuse were similar to those of healthy women with neither an abuse history nor psychiatric illness.
The findings may explain both positive and negative findings in previous studies of hippocampal volume in major depressive disorder. The finding of smaller hippocampal volumes in depressed women with abuse in this study is similar to reports of smaller hippocampal volumes in some studies of patients with major depressive disorder
(5,
14–17). The normal hippocampal volume in depressed women without abuse in this study is similar to reported negative findings in earlier studies
(18–
20,
22,
23). Close examination of the MRI studies of patients with major depressive disorder suggests that hippocampal structural abnormalities may be restricted to subjects with treatment-resistant depression
(15,
49), older women with major depressive disorder
(5,
16), women with treatment-resistant depression
(18), and elderly depressed patients
(16,
17), with some exceptions
(14,
19,
20). Patients with depression and comorbid illnesses such as PTSD are known to have a poorer response to antidepressants, compared to patients with depression alone. Findings of smaller hippocampal volumes in depressed women and in patients with treatment-resistant depression but not in mixed groups of patients with treatment-responsive depression raise the possibility that prepubertal childhood physical and/or sexual abuse could be associated with poor antidepressant response as well as with morphological abnormalities in the hippocampus in adulthood. Because, to our knowledge, no previous studies of hippocampal volume in major depressive disorder have documented the presence or absence of childhood physical and/or sexual abuse, it is possible that varying trauma histories may have contributed to the disparate findings in hippocampal morphometry. Hippocampal abnormalities could be a direct consequence of severe childhood trauma or could occur as a result of interaction with other factors, such as depression, alcohol use, or medical illness. In this study, however, significant differences in hippocampal volumes between depressed subjects with childhood trauma and comparison subjects were found after controlling for a past history of alcohol and other substance abuse.
The findings of this study are similar to those of a study by Stein et al.
(28) reporting smaller left hippocampal volumes in women with childhood sexual abuse, although the magnitude of the difference in hippocampal volume was greater in the present study (18% smaller mean volume versus 5% smaller mean volume than in normal comparison subjects). Because Stein et al.
(28) did not include a depressed group without abuse and only a third of the 21 subjects in the current study had childhood abuse and current major depression, definitive conclusions regarding the contribution of early trauma to hippocampal volume in depressed adults could not be drawn. Smaller hippocampi have been reported in adult subjects with PTSD secondary to combat
(47,
50) and to childhood physical and/or sexual abuse
(27). In contrast, similar smaller hippocampal volumes have not been found in children with PTSD secondary to sexual abuse
(51,
52) and in adults with PTSD after a motor vehicle accident
(53). Taken together, MRI studies in PTSD suggest that the severity, duration, frequency, and kind of trauma as well as the subject’s age during exposure to trauma and comorbid alcohol use
(54) could contribute to the contradictory findings about hippocampal volume in this disorder. We attempted to reduce variability in the study group by restricting inclusion to women with a history of severe, recurrent, and prolonged abuse that occurred before puberty. The volume of the left hippocampus was smaller than normal in this study as well as in prior studies that evaluated brain changes related to childhood trauma
(27,
28). In contrast, smaller right hippocampal volumes were seen in subjects with postpubertal traumatic experiences
(46,
49). The possibility that traumatic experiences before puberty may differentially affect the developing left hippocampus, particularly the head, needs to be examined.
The absence of a comparison group with early childhood trauma without current major depressive disorder or PTSD limits our ability to determine whether having a smaller left hippocampus is a risk factor for developing major depressive disorder or a consequence of abuse. Numerous preclinical studies support the possibility that morphological changes in the hippocampus are a consequence of stress. Exposure to chronic psychosocial stress and administration of hydrocortisone alter the morphology of apical dendrites and the synaptic structure of hippocampal neurons (reviewed in references
12,
55). Psychosocial stress, as well as glucocorticoids, inhibits neurogenesis in the dentate gyrus of the hippocampus
(9,
56). Recent preclinical evidence supports the possibility that exposure to elevated levels of corticotropin releasing hormone (CRH) early in development results in progressive loss of hippocampal CA3 neurons
(57). Increased levels of CSF CRH seen in rats after maternal separation
(58–
60), in nonhuman primates exposed to variable foraging demand
(61), and in humans with PTSD
(62,
63) raise the possibility that elevated CRH levels could have a direct neurotoxic effect on hippocampus neurons in stress-related disorders.
Human studies have replicated increased HPA axis sensitization after childhood abuse. Women with current depression and a history of childhood physical and/or sexual abuse had greater increases in plasma cortisol and ACTH in response to the Trier Social Stress Test
(39). The importance of childhood abuse is further supported by the finding that subjects with major depressive disorder and without a history of childhood abuse had a normal cortisol response to a psychological stress paradigm
(39,
64, unpublished 2002 manuscript of M. Vythilingam et al.). A similar enhancement of pituitary response to an endocrine challenge test—the CRH stimulation test—was seen in depressed children with ongoing abuse
(65) and in women with childhood abuse
(40), suggesting that persistent abnormalities in the HPA axis occur relatively early in life. Thus, increased levels of CRH and cortisol during repeated childhood abuse together with persistent hyperactivity and sensitization of the HPA axis in adulthood could damage hippocampal neurons in adult women with major depressive disorder. The lack of a smaller hippocampal volume in depressed women without a history of abuse in this study as well as in an independent sample of depressed subjects recruited at a different center (unpublished 2002 manuscript of M. Vythilingam, et al.) further highlights the deleterious effects of childhood abuse on brain development.
Smaller hippocampal volume could also be a risk factor for developing psychiatric disorders after exposure to overwhelming stress. Recent studies in humans and in nonhuman primates confirmed that about 40%–54% of the variance in hippocampal volume is genetically determined
(66,
67). Morphological changes in the hippocampus have not been consistently observed subsequent to psychosocial stress or hydrocortisone administration
(68,
69). Gilbertson et al.
(70) observed smaller hippocampal volume both in non-combat-exposed and in combat-exposed identical co-twins with PTSD and concluded that smaller hippocampi increase the vulnerability for developing PTSD in individuals exposed to trauma. Similar twin studies or prospective studies in high-risk populations will help resolve whether smaller hippocampal volume predisposes traumatized individuals to developing depression.
We demonstrated that smaller left hippocampal volume in women with major depressive disorder can be attributed to severe and repetitive physical and/or sexual abuse in childhood. Given the high prevalence of abuse in childhood, future studies evaluating hippocampal structure and function in depressed subjects should treat subjects with and without childhood abuse as separate groups. This strategy will help to increase homogeneity among subjects and to enhance reproducibility of biological findings in psychiatric illnesses. Future research should also focus on developing strategies to prevent and reverse structural brain changes after exposure to traumatic experiences in childhood.