Suicide is a major public health concern
(1) and the ninth leading cause of death; about 30,000 individuals die by suicide each year in the United States alone
(2). In the young, it is the second most frequent cause of death. Suicidal behavior is often associated with mental disorders, such as depression, bipolar illness, and personality disorders. Besides psychiatric illnesses, other risk factors include a family history of suicide, a family history of psychiatric disorders, psychosocial stressors, impulsivity, and aggression
(3). Abnormalities in neurobiological mechanisms may be another risk factor for suicide. Several studies
(4,
5) have suggested that the major driving factor leading to suicide in teenagers is aggressive and impulsive behavior. Since abnormalities in serotonin (5-HT) function have been implicated in impulsive/aggressive behaviors
(6), it is possible that the pathophysiology of teenage suicide may be associated with abnormal serotonin function.
Studies of postmortem brain samples from adult suicide victims have suggested that abnormalities of 5-HT receptor subtypes are associated with suicide (for a review, see Gross-Isseroff et al.
[7]). In particular, it has been reported by several investigators, although not confirmed by all, that one of the serotonin receptor subtypes, the 5-HT
2A receptor, is found in higher than normal levels in the postmortem brains of adult suicide victims
(7–
10). Further support for this concept comes from studies that indicate a greater number of 5-HT
2A receptors in the platelets of suicidal patients with different mental disorders; this higher level appears to be independent of diagnosis
(11,
12). On the basis of these findings, we previously proposed that platelet 5-HT
2A receptors might serve as a biological marker of suicidal behavior
(11).
Method
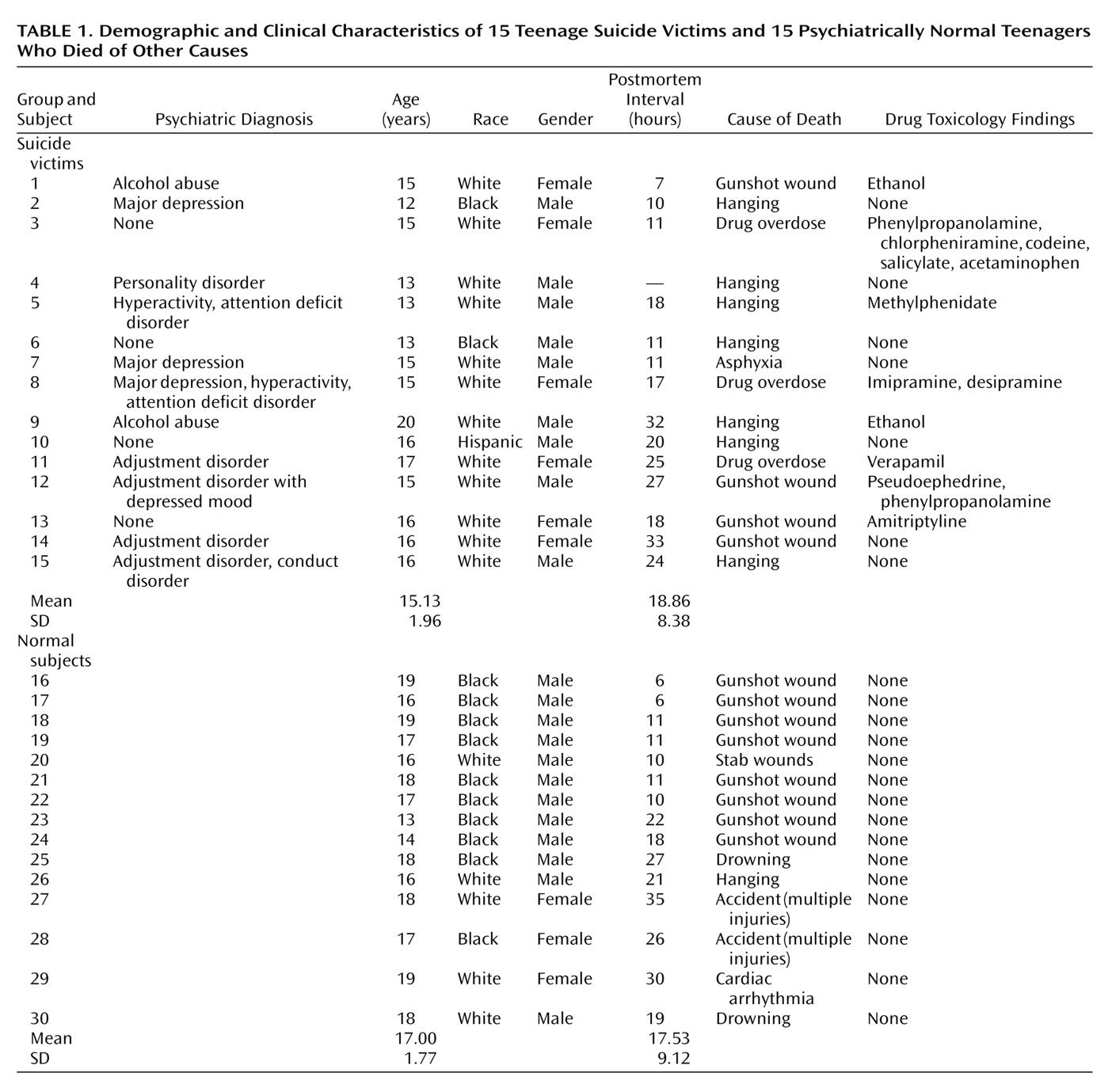
5-HT2A receptor levels were determined in the prefrontal cortex (Brodmann’s areas 8/9), hippocampus, and nucleus accumbens (all from the right hemisphere) in brains obtained from 15 teenage suicide victims and 15 psychiatrically normal teenage subjects who died of other causes, herein referred to as normal subjects. Postmortem brain tissue was obtained from the Brain Collection Program at the Maryland Psychiatric Research Center in Baltimore, Md., in collaboration with the Medical Examiner’s Office of the State of Maryland. Brain samples were free of neuropathological abnormalities and HIV antibodies. Toxicological data were obtained by analysis of urine and blood samples.
Diagnostic Methods
All study victims were diagnosed by means of the Structured Clinical Interview for DSM-III-R
(13) and the Diagnostic Evaluation After Death
(14); diagnoses were based on the information obtained from interviewing at least one family member and from all available medical records. Discrepancies were resolved through a consensus conference between two senior psychiatrists. Subjects were considered to be suicides only if the manner of death was determined to be suicide by the medical examiner. The comparison groups consisted of normal subjects who were verified as free from mental illnesses by use of these same diagnostic procedures. This study was approved by the institutional review board at our facility.
[125I]LSD Binding to 5-HT2A Receptors
Tissues were homogenized in 10 vol of hypotonic medium (5 mM of Tris and 0.1% of EDTA; pH=7.5), by using a polytron setting of 9 for 30 seconds, and centrifuged at 1,000 g for 10 minutes at 4°C. The resulting supernatant was centrifuged at 30,000 g for 15 minutes at 4°C. The pellet thus obtained was washed twice with 10 vol of hypotonic buffer and centrifuged. The final pellet was suspended in 2–5 ml of incubation buffer (50 mM of Tris, 120 mM of sodium chloride, 5 mM of potassium chloride, 1 mM of magnesium chloride, and 0.05% of ascorbic acid; pH=7.4), depending on the size of the membrane pellet. This suspension was used for the determination of the 5-HT2A receptor level.
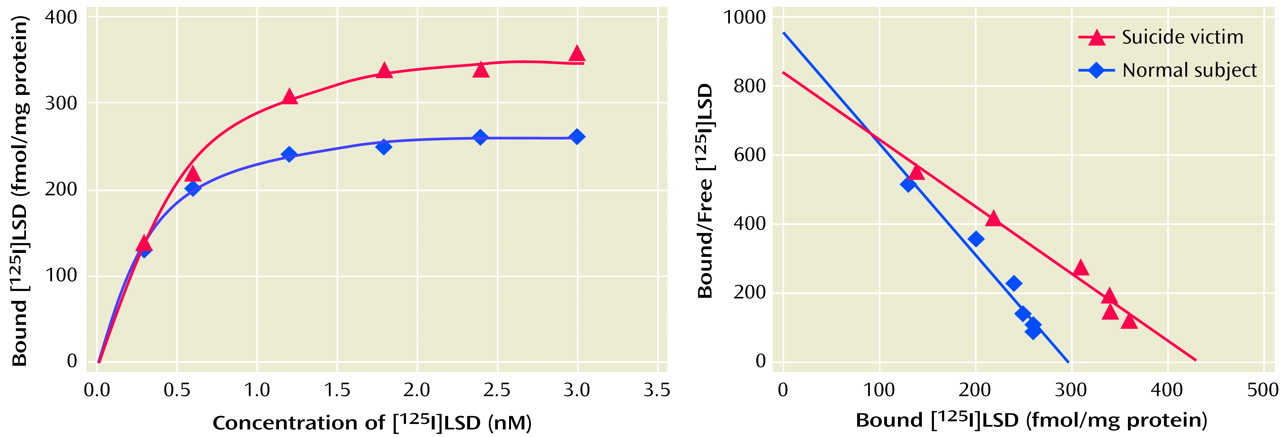
The receptor binding assay was carried out in triplicate in plastic tubes containing incubation buffer, [125I]LSD, ranging from 0.25–3.00 nM (five to six different concentrations), and a 40-μl cortical membrane suspension with or without 1 μM of ketanserin in a total incubation volume of 100 μl and incubated at 37°C for 1.5 hours. The incubation was terminated by rapid filtration over glass microfiber filters and washed three times with 5 ml of ice-cold 50-mM Tris buffer (pH=7.7) containing 0.01% bovine serum albumin. The filters were dried and then counted by a gamma counter. Specific binding was defined as the difference between the binding observed in the presence or absence of 1 μM of ketanserin and ranged from 75% to 80%, depending on the concentration of the ligand.
Polymerase Chain Reaction Analysis
The mRNA content of the 5-HT
2A receptors was measured by use of quantitative reverse transcription polymerase chain reaction, as described earlier
(15), with internal standards and these amplification primers: forward, 772–779 bp (5′ATCAAGTCACTCCAGAAAGAAGCT); reverse, 1153–1176 bp (5′TGAAAAGGCTGACCTATAGGTCTT).
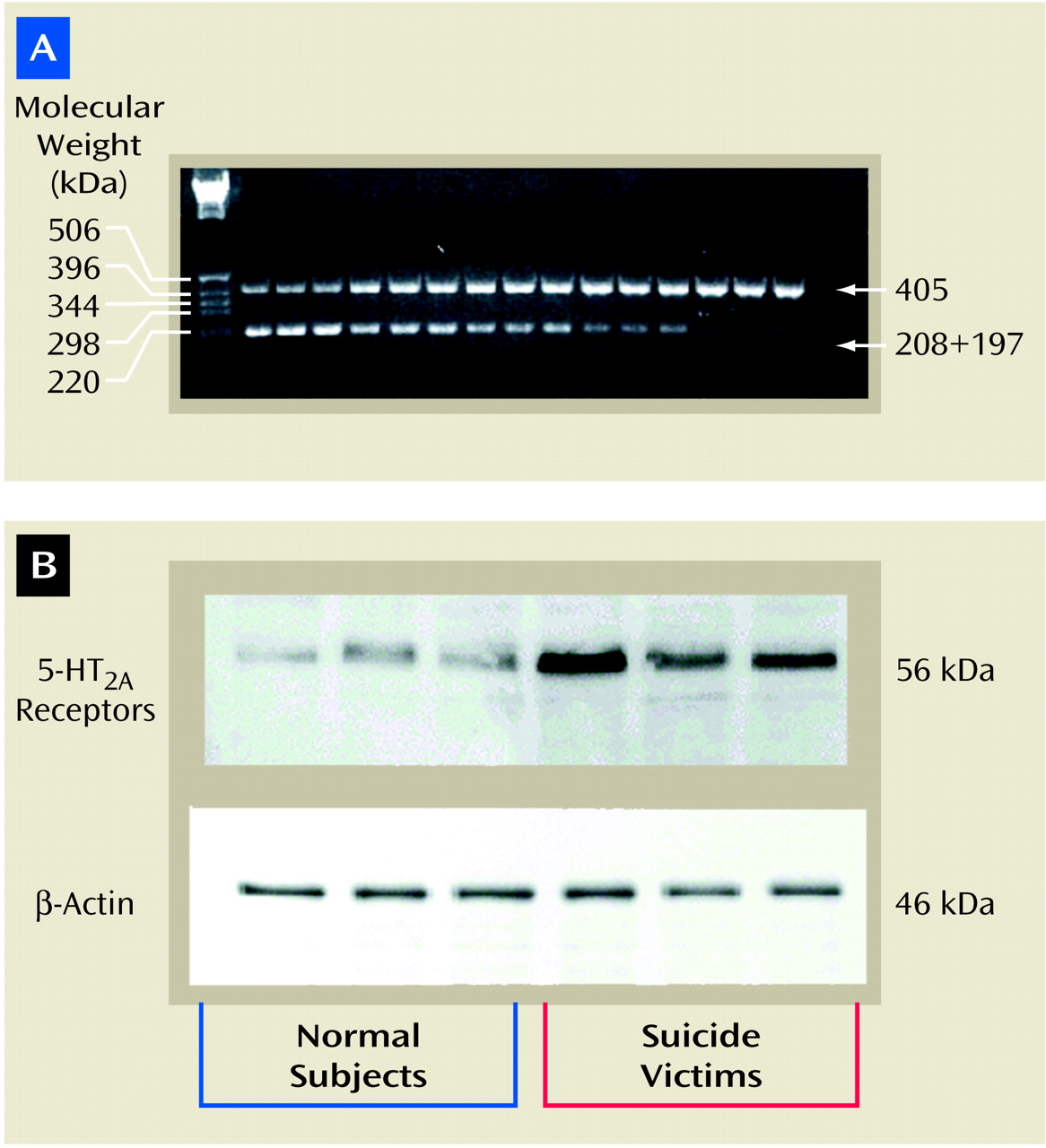
Internal standard templates were generated by site-directed mutagenesis to introduce a BglII restriction site midway between the amplification primers so that the digestion of internal standard amplicon would generate two fragments of approximately equal size (208 and 197 bp). The internal primer sequence was as follows: bp=958–981, 5′AAGGCATGCAAGATCTGGGCATC. The italicized bases indicate the BglII restriction site, while bold and italicized bases [bold not shown; “ATC”] indicate the mutation site. To quantitate 5-HT2A receptor mRNA, various amounts of cRNA were added to a l-μg mixture of total RNA and RNA–cRNA and reverse transcribed by using 200 U of Moloney’s murine leukemia virus reverse transcriptase. The transcription was carried out in the presence of random hexamer, 50 mM of Tris hydrochloride (pH=8.3), 74 mM of potassium chloride, and 3 mM of magnesium chloride in a volume of 20 μl. The incubation was carried out at room temperature for 10 minutes then at 37°C for 1 hour; the contents were heat denatured at 95°C for 5 minutes. The aliquots were amplified with 1 μM of primers, 200 mM of deoxynucleotide triphosphate, 1.5 mM of magnesium chloride, 50 mM of Tris hydrochloride (pH=9.0), 20 mM of ammonium sulfate, 1 μCi of [32P]cytidine 5′-triphosphate, and 2.5 U of Hot Tub DNA polymerase (Amersham Pharmacea Biotech, Piscataway, N.J.) in a total volume of 100 μl. The mixture was amplified for 30 cycles: 94°C for 45 seconds, 60°C for 1 minute; 72°C for 1 minute, and 72°C for 15 minutes. Fifty-one microliters of aliquots were digested with 30 U of BglII overnight at 37°C in 50 mM of Tris hydrochloride (pH=8.0), 10 mM of magnesium chloride, and 100 mM of sodium chloride in a volume of 60 μl. The competitive polymerase chain reaction was analyzed by agarose gel electrophoresis in triplicate (1.5% weight/volume) in 0.5 × Tris-borate-EDTA buffer.
To quantitate the amount of product, bands stained with ethidium bromide were removed and counted. Data are presented as the counts incorporated into the amplified cRNA standard, divided by the counts incorporated into the corresponding subunit mRNA amplification product, versus the counts from a known amount of internal standard (cRNA) added to the test sample.
Determination of Protein Levels
Tissues were homogenized in homogenizing buffer containing 5 mM of Tris hydrochloride (pH=7.4), 0.1% of EDTA, 2 μM of leupeptin, 0.5 mM of phenylmethylsulfonyl fluoride, 1.45 μM of pepstatin, 0.2 U/ml of aprotinin, and 2 mM of dithiothreitol. The homogenate was centrifuged at 3,000 rpm for 10 minutes at 4°C. The supernatant was recentrifuged at 32,000 rpm for 15 minutes at 4°C. The resulting pellet was resuspended in homogenizing buffer.
The samples (30 μg of protein in each lane) were resolved on 7.5% polyacrylamide gel and then blotted for 2 hours on enhanced chemiluminescence membranes. The membranes were then incubated overnight with 5-HT2A receptor antibody (1:5000 dilution; Pharmingen, San Diego) and thereafter with a horseradish peroxidase-linked secondary antibody (antimouse immunoglobulin G [IgG], 1:3000 dilution) for 5 hours. The signal was detected by treating the blots with Western blot detection reagent (Amersham Pharmacia Biotech, Piscataway, N.J.) and exposing them to enhanced chemiluminescent membrane. To determine the immunolabeling of β-actin, the membranes were stripped and incubated with β-actin (monoclonal β-actin antibody) at a dilution of 1:3000 for 5 hours and with secondary antibody (antimouse IgG) at a dilution of 1:5000 for 2 hours. The optical densities of the bands were quantified by using a densitometer (Loats Image Analysis System, Westminster, Md.), and values were corrected with the optical density of β-actin protein in each sample.
Gold Immunolabeling
Brain tissue samples that were flash frozen after autopsy and kept at –80°C were dropped into ice-cold fixative (4% paraformaldehyde in 0.1 M of phosphate-buffered saline; pH=7.4) and rotated for 2 hours at 4°C. Tissues were kept in fixative at 4°C for 24 hours, embedded in 30% sucrose in phosphate-buffered saline, and refrozen on dry ice for sectioning.
Twenty-micron sections were rinsed in phosphate-buffered saline for 2 days at 4°C and blocked with RPMI medium 1640 (Gibco-BRL, Grand Island, N.Y.) for 30 minutes. This was followed by rinsing in 2% normal serum for 30 minutes and 1% bovine serum albumin in phosphate-buffered saline for 30 minutes before incubation in 5-HT2A receptor antibody (2 μg/ml) diluted in 1% bovine serum albumin in phosphate-buffered saline for 18 hours at 4°C, followed by 2 hours at room temperature, while rotating. Sections were rinsed twice in 1% bovine serum albumin in phosphate-buffered saline for 30 minutes, then incubated for 60 minutes (at room temperature) with the corresponding secondary antibody conjugated to l nm of colloidal gold particles and diluted in 1% bovine serum albumin in phosphate-buffered saline (concentration of 1:200). Sections were then rinsed several times for 30 minutes with 1% bovine serum albumin in phosphate-buffered saline, followed by distilled water. For gold particles to be visible with light microscopy, they were silver-enhanced for 12 minutes and rinsed repeatedly with distilled water. For comparison sections, from which nonspecific labeling was determined, an identical protocol was followed, except that 1% bovine serum albumin in phosphate-buffered saline was substituted for the primary antibody. Sections were then mounted onto microscope slides, air dried, and counterstained with toluidine blue.
Quantification of Gold Immunolabeling
For quantification purposes, an oil immersion objective of 100× was coupled with a 1.6× magnification tube; therefore, the number of immunogold particles was clearly discernible. When there is dense immunolabeling, immunogold particles sometimes overlap. Particles are counted by using a Samba-2000 image analysis system (Imaging Products International, R&M BioMetrics, Inc., Chantilly, Va.). The grain-counting program first calculates the average size of a silver-enhanced gold particle, then quantifies the number of gold particles on the basis of their average size. For each area, gold particles were also quantified manually by an experimenter who was blind to the diagnosis to ensure the reliability of the computerized counts. An interclass correlation coefficient (ICC) with 95% confidence intervals (CIs) for the reliability of computer-generated counts, as compared to manual counts, for gold particle quantification were as follows: pyramidal cells: ICC=0.9177 (95% CI=0.8194–0.9625) for normal subjects and ICC=0.9364 (95% CI=0.8531–0.9725) for suicide victims; neuropil: ICC=0.9015 (95% CI=0.7839–0.9551) for normal subjects and ICC=0.9054 (95% CI=0.7814–0.9591) for suicide victims.
With the Samba image analysis, 120×120-μm sampling frames were randomly placed in each cortical layer. For pyramidal cells, only cells that met the following criteria were measured: 1) presence of a nucleolus, 2) having a pyramidal shape, and 3) presence of an apical dendrite. The average number of gold particles per 100 μm2 was calculated in the cell soma of at least 10 randomly selected cells in each cortical layer for each of three to five 20-μm sections of the prefrontal cortex. The mean coefficients of error for average number of gold particles per cell was 2.8% (SD=0.5%) for the normal subjects and 3.2% (SD=0.6%) for the suicide victims. To estimate gold immunodensity in the neuropil, we averaged at least 10 measurements taken in randomly selected 100-μm2 cell-free regions of each cortical layer of three to five 20-μm sections. The mean coefficients of error for average number of gold particles per 100 μm2 of cell-free neuropil region were 1.3% (SD=0.2%) for the normal subjects and 1.1% (SD=0.3%) for the suicide victims. Nonspecific labeling was determined by counting the number of gold particles per 100 μm2 in sections in which the primary antibody was omitted. Background labeling was corrected by subtracting nonspecific labeling on pyramidal cells from the total labeling on pyramidal cells and likewise by subtracting nonspecific labeling in the neuropil from total labeling in the neuropil.
Statistical Analyses
Data analyses were performed by using the SPSS 8.0 statistical software package (SPSS, Inc., Chicago). [125I]LSD binding, protein, and mRNA 5-HT2A receptor levels were compared between suicide victims and normal subjects by using analysis of covariance, in which race was used as covariate. Group differences between normal subjects and suicide victims with or without a history of mental disorders were tested in one-way analysis of variance, followed by Bonferroni’s multiple comparison procedure where significant main effects were present. The relationships among postmortem interval, age, and 5-HT2A receptor measures were determined by Pearson product-moment correlation analyses. Comparisons of 5-HT2A receptor measures with gender or race were performed with independent t tests. Age and postmortem interval were compared between suicide victims and normal subjects with independent t tests. The level of significance was alpha ≤0.05.
Discussion
We have examined the role of 5-HT
2A receptors in teenage suicide, using postmortem brain samples. Although it is not clear if the etiology or the factors underlying teenage suicide are similar to those found in adult suicide, several factors lend credence to a relationship between serotonin and teenage suicide. Most teenage suicide is driven by impulsive or aggressive behavior, stress, or anxiety, which have been shown to be related to abnormalities in serotonergic mechanisms
(6); hence, it is quite likely that teenage suicide may be related to abnormalities in serotonergic mechanisms.
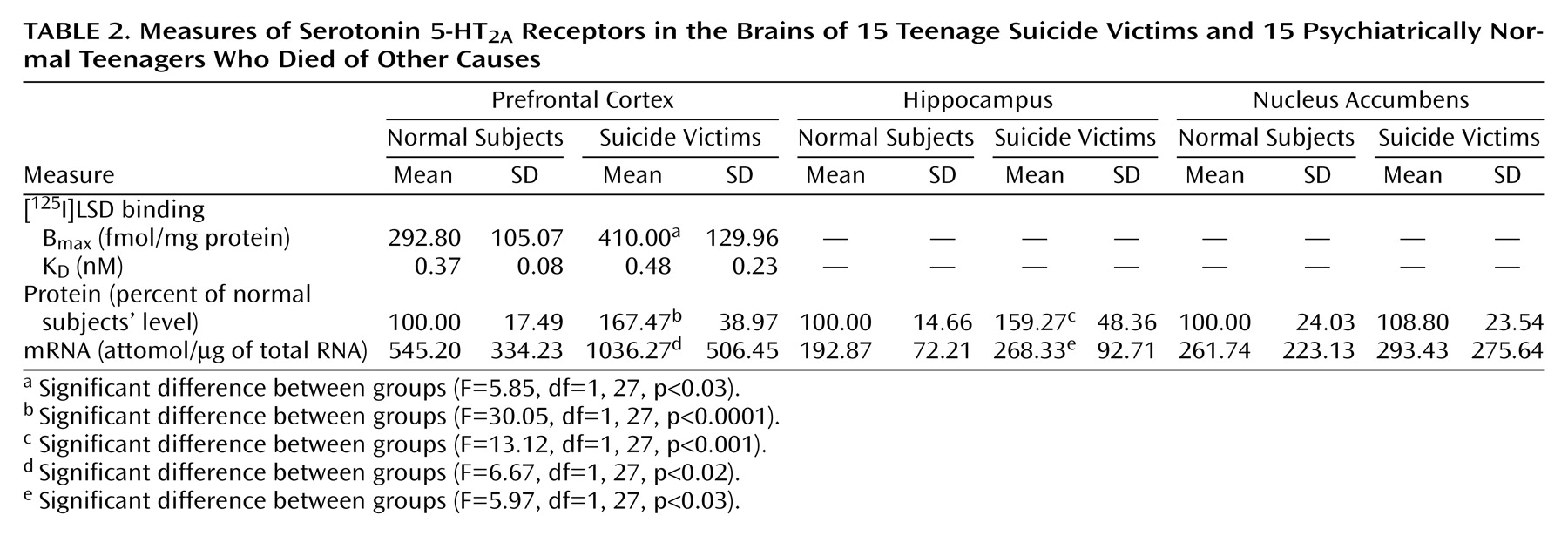
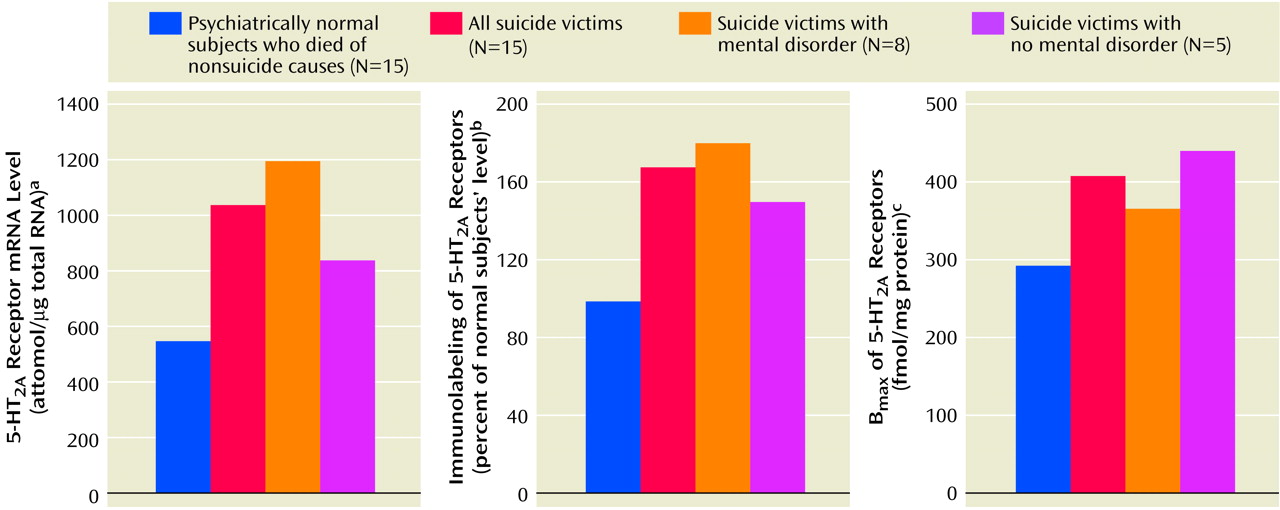
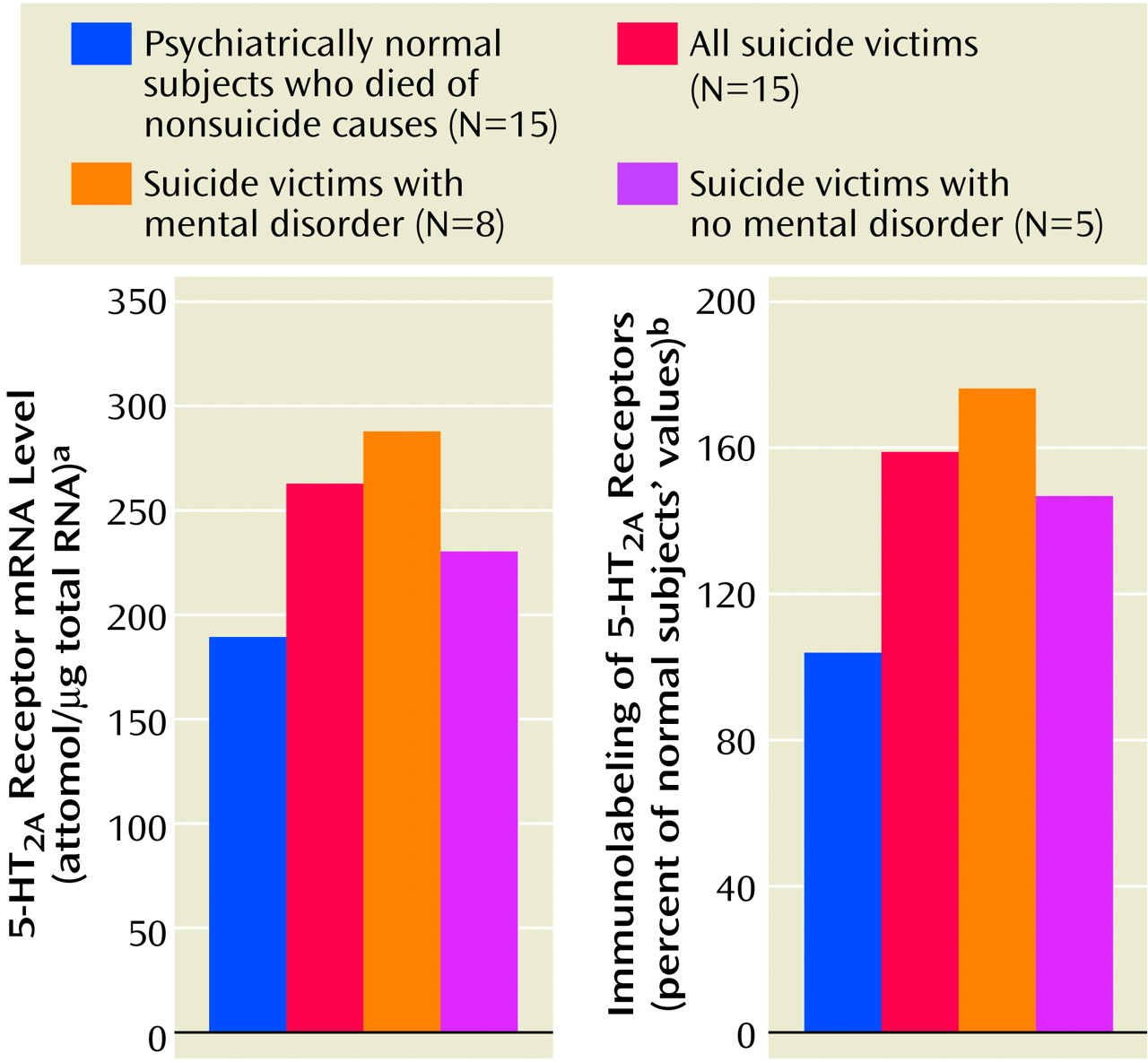
We found a striking difference in 5-HT
2A receptors between teenage suicide victims and normal subjects. First, we observed that 5-HT
2A receptor binding sites are more numerous in the prefrontal cortex of teenage suicide victims than in normal subjects. The observation of a higher level of 5-HT
2A receptors in the prefrontal cortex of teenage suicide victims is similar to that observed in adults in previous studies
(7). However, we also observed a higher gene expression of 5-HT
2A receptors and of its cognate protein in the prefrontal cortex and hippocampus, but not in the nucleus accumbens, in the postmortem brains of teenage suicide victims than in normal subjects. These differences were not related to postmortem interval or age, and the absence of correlation between age and 5-HT
2A receptors could be due to the narrow age range of our subjects.
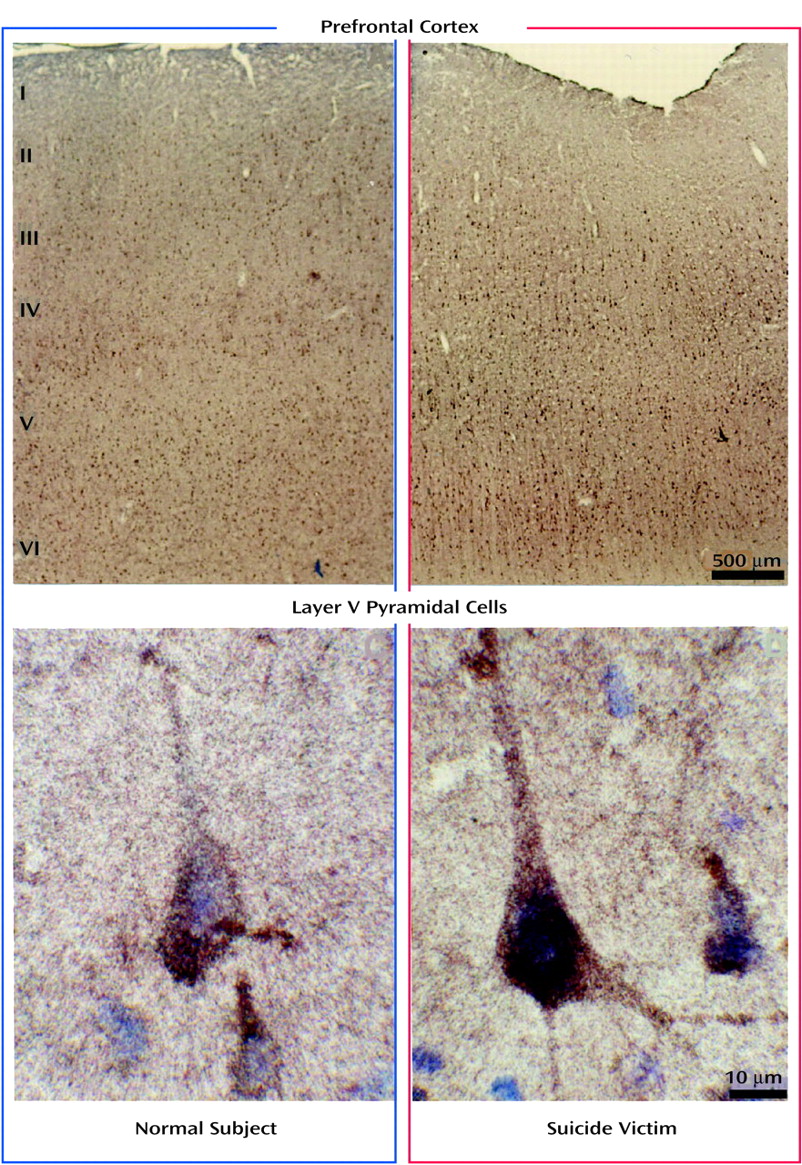
Since it has recently been reported that 5-HT
2A receptors are highly expressed in the pyramidal neurons in cortical layers III, V, and VI of the primate and rat brain
(18,
19), we determined whether the observed higher protein levels of 5-HT
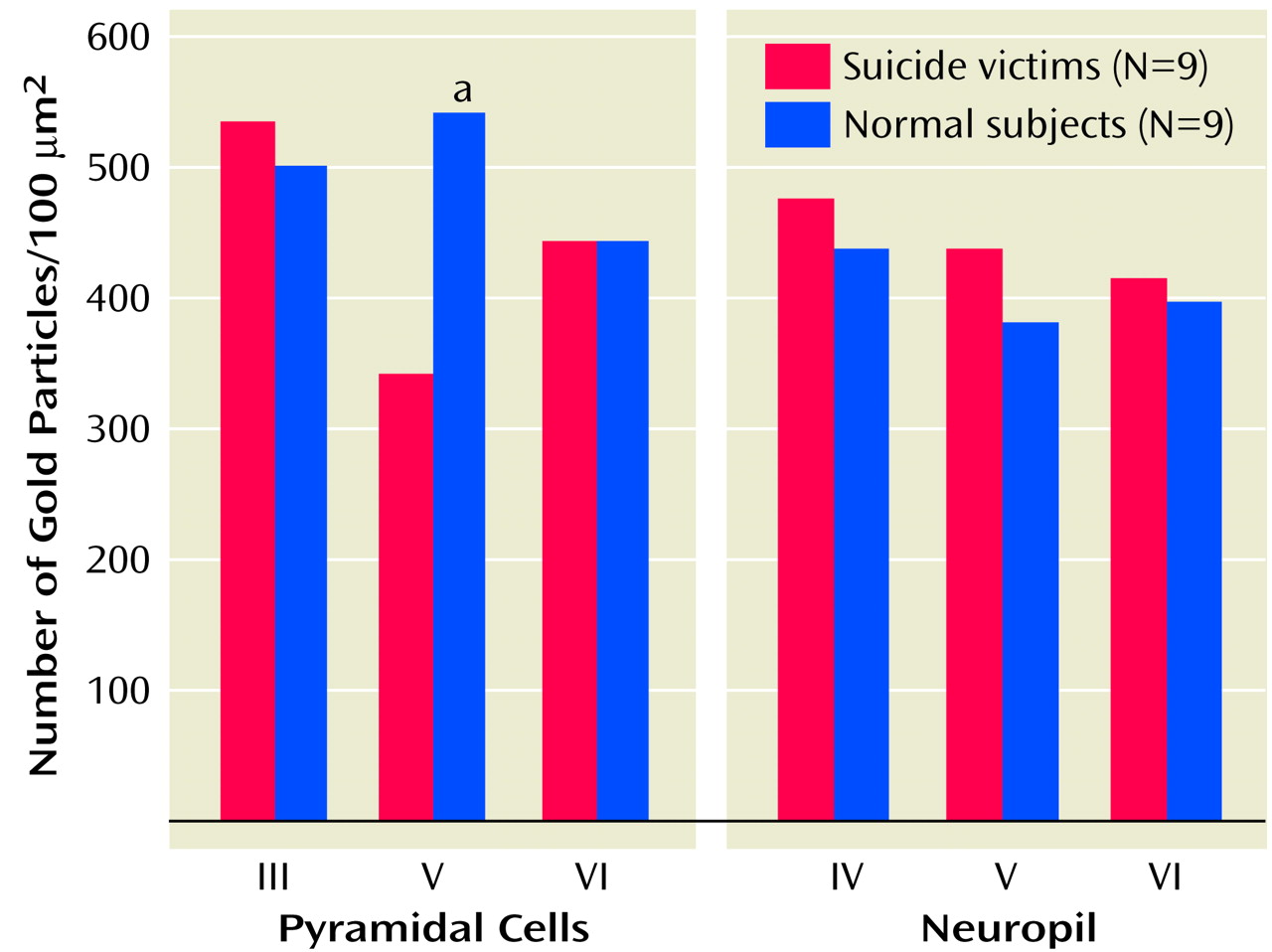
2A receptors were restricted to these specific areas or whether this change was localized throughout all the cortical layers. Similar to findings in the primate brain, in this study we found that 5-HT
2A receptors were densely expressed in pyramidal neurons in layers III, V, and VI and to a lesser extent in the surrounding neuropil. Our comparison of the expression of 5-HT
2A receptors in various cortical layers of the brains of teenage suicide victims and normal subjects led to an interesting observation that 5-HT
2A receptor protein expression is higher only in the pyramidal cells of cortical layer V but not in cortical layers III or VI or in the surrounding neuropil. Our findings thus show that a greater number of active binding sites of 5-HT
2A receptors is not merely a consequence of more binding sites but also that gene expression contributes to this greater number. More important, this higher level is restricted to a specific cortical layer. This is an important observation because pyramidal neurons occupy a unique position as they modulate and integrate neuronal functions mediated by serotonergic, glutamatergic, GABA-ergic, and dopaminergic systems
(20). It is also important because it has been shown that the soma and dendrites of pyramidal neurons of layer V receive synaptic contact from dopamine terminals and GABA-containing neurons. Since it has been shown that GABA-ergic mechanisms, including benzodiazepine receptors, may be altered in the postmortem brains of suicide victims
(21), it is quite possible that higher levels of 5-HT
2A receptors in pyramidal cells may cause an imbalance between the 5-HT
2A and the GABA-ergic systems and may play an important role in suicidal behavior. Jakab and Goldman-Rakic
(20) wrote that “the apical dendritic field proximal to the pyramidal cells soma is the hot spot for 5-HT
2A receptor mediated physiological actions relevant to neuronal and psychotic functional states of the cerebral cortex.” They also observed from in vivo recordings in the prefrontal cortex of monkeys that 5-HT facilitates excitatory neurotransmission in the pyramidal neurons engaged in information processing.
Another important observation of this study pertains to the higher 5-HT
2A receptor expression specifically in the prefrontal cortex and hippocampus but not in the nucleus accumbens of suicide victims. The prefrontal cortex and hippocampus have been implicated in emotion, stress, and cognitive functions; the nucleus accumbens has been associated with craving, drug dependence, and psychomotor-stimulated behavioral sensitization
(22). Both emotion and stress play important roles in suicidal behavior, especially in youth. The higher 5-HT
2A receptor indices specifically in the prefrontal cortex and hippocampus thus further strengthen the association of higher levels of 5-HT
2A receptors and suicidal behavior.
5-HT
2A receptors have been previously studied in the postmortem brains of adult suicide victims with depression and also in nonsuicidal schizophrenia victims. Whereas higher levels of 5-HT
2A receptors have been reported in depressed suicide victims
(9), a lower number of 5-HT
2A receptor binding sites and lower mRNA levels have been reported in patients with schizophrenia
(23). From these studies, it is not clear if higher 5-HT
2A receptor levels are associated with suicide, depression, or suicidal schizophrenia, since insufficient data exist for suicidal schizophrenia patients. In our study of teenage suicide victims, we examined the relationship of suicide and/or mental disorders with 5-HT
2A receptors. Since the numbers of victims with specific psychiatric diagnoses was small, we grouped all victims with a history of mental disorders together, including victims with mood and/or adjustment/conduct disorders. A significant number of victims had no history of mental disorders. Since we did not find any significant differences in any of the 5-HT
2A receptor measures (mRNA, protein expression, or binding) between suicide victims with or without a history of mental disorders in any of the brain areas we studied, it appears that the higher level of 5-HT
2A receptors is specific to suicide and most likely independent of psychiatric diagnosis. However, these 5-HT
2A receptor levels were still significantly higher in both of these groups, compared with the normal subjects. Although this finding is consistent with our previous report that the higher level of platelet 5-HT
2A receptors observed in suicidal victims is independent of diagnosis
(11), confirmation in a larger number of suicide victims with specific diagnoses of psychiatric disorders is needed. It is also possible that in teenage suicide victims, higher levels of 5-HT
2A receptors may be related to impulsive-aggressive behavior. However, this could not be ascertained in this study group, since no data regarding aggressive/impulsive behavior in these victims were available.
The reasons for the higher levels of 5-HT
2A receptors in suicide victims are not known, and it is unclear if higher levels of 5-HT
2A receptors in the brains of suicide victims indicate a higher vulnerability for suicide. In a recent study, Du et al.
(24) examined the 5-HT
2A receptor gene in platelets obtained from suicidal and nonsuicidal depressed patients and normal comparison subjects. They found a significant association between the 102C allele in the 5-HT
2A receptor gene and major depression, particularly in patients with suicidal ideation. Since the same gene encodes both brain and platelet 5-HT
2A receptors
(25), our observation of higher 5-HT
2A receptor levels in the prefrontal cortex may represent a greater genetic vulnerability for suicide.
However, it is possible that the higher 5-HT
2A receptor levels in suicide victims is secondary to changes in other systems, such as abnormalities in the hypothalamic-pituitary-adrenal (HPA) axis. Abnormal HPA axis function and higher levels of cortisol have been consistently observed in patients with depression
(26,
27) and suicidal behavior
(28,
29). Interaction between the HPA axis and the serotonergic system has also been clearly demonstrated (reviewed by Chauloff
[30], Kuroda et al.
[31], Fernandes et al.
[32], and Mikuni et al.
[33]). Moreover, it has been shown that the administration of dexamethasone or corticosterone causes an increase in 5-HT
2A receptor levels in the rat cortex
(31). Recently, Farisse et al.
(34) demonstrated that transgenic mice bearing a transgene coding for glucocorticoid receptor antisense mRNA, which partially blocks glucocorticoid receptor expression, show lower glucocorticoid receptor numbers and a higher level of 5-HT
2A receptors. Since cortisol levels are higher in patients with depression and during periods of stress or anxiety, the higher levels of 5-HT
2A receptors in the brain of suicide victims may be due to an abnormal HPA axis. It is also quite possible that genetic and environmental factors (higher cortisol level) may in combination cause up-regulation of cortical 5-HT
2A receptors.
In summary, this is the first study to our knowledge that demonstrates higher levels of gene expression of 5-HT2A receptors and of the encoded 5-HT2A receptor protein in the prefrontal cortex of suicide victims (either teenage or adult); it shows that these higher levels are restricted to the pyramidal cells of layer V, which are considered a “hotspot” for 5-HT2A receptor-mediated physiological actions. This higher level of 5-HT2A receptors may play a vital role in suicidal behavior and may represent a greater risk for suicide.