Omega-3 fatty acids have received considerable attention as a therapeutic option in cardiovascular disease
(1). These compounds are derived from fish oil, and epidemiologic evidence suggests some relationships between ingestion of fish oil and both cardiac disease and depressive disorder in different cultures. Studies have reported that countries with high rates of fish oil consumption have low rates of depressive disorder
(2). One controlled double-blind trial
(3) found marked therapeutic efficacy and no side effects of omega-3 fatty acids in the prevention of bipolar manic-depressive illness.
Several studies of omega-3 fatty acids have been performed in behavioral disorders and have found few side effects
(4). There is evidence suggesting that omega-3 has an effect on human CSF serotonin metabolites
(5). Edwards et al.
(6) reported changes in fatty acid levels in the diet and red blood cells of depressed patients. Maes et al.
(7) reported changes in serum fatty acid composition in depressive disorder. Severus et al.
(8) proposed that omega-3 fatty acids are the mechanistic “missing link” connecting cardiovascular disease and depressive disorder, representing a key pathophysiological clue to the mechanism of depressive disorder.
We studied a specific omega-3 fatty acid, the ethyl ester of eicosapentaenoic acid (E-EPA), as an adjunct to antidepressant treatment for episodes of depressive disorder occurring in patients with recurrent unipolar depressive disorder who were receiving maintenance antidepressant therapy.
Method
Patients with current major depressive disorder diagnosed according to DSM-IV were included in the study if they were 18–75 years old, had no unstable medical disease, no alcohol or drug abuse, no psychotic features, no history of hypomania or mania, and no comorbid psychiatric diagnosis other than panic disorder, dysthymic disorder, or obsessive-compulsive disorder (OCD). All patients participated in at least two clinical interviews with at least two specialist psychiatrists separated by at least 1 week before consensus diagnosis.
After baseline physical examination and determination of blood chemistry, patients were randomly assigned to receive E-EPA or placebo according to a prearranged code. The E-EPA (derived from 96% pure fish oil) and matching placebo were supplied by Dr. David Horrobin, Laxdale Ltd. (Stirling, U.K.). The other fatty acids making up the remaining 4% were those typically present in fish oil. The fish oil was stabilized with 0.2% vitamin E and provided in 500-mg soft gelatin capsules. Fish taste and smell were minimal.
This study was approved by the institutional review board of the Ministry of Health Mental Health Center, Faculty of Health Sciences, Ben Gurion University of the Negev, Beer-Sheva, Israel. All participating patients gave written informed consent. The review board had approved a trial of E-EPA versus placebo added to standard antidepressant treatment of any patient with depressive disorder. Clinical practice, however, led to our including only patients who had experienced episodes of depressive disorder while receiving antidepressant treatment, with the exception of one patient (number 19) who was not receiving maintenance antidepressant treatment but had a 4-month severe depressive disorder with comorbid OCD that was resistant to two selective serotonin reuptake inhibitors. With the exception of this patient, all patients had been receiving their current antidepressant treatment for at least 3 months.
The design was a 4-week, parallel-group, double-blind addition of E-EPA or placebo to ongoing antidepressant therapy. Patients continued their current antidepressant treatment at the same dose they were receiving when they entered the study. Patients included in the study had been receiving their antidepressant medication for at least 3 weeks at the current therapeutic dose. The patients’ baseline scores on the 24-item Hamilton Depression Rating Scale were 18 or higher. The E-EPA or matching placebo was given in 1-g doses twice a day for a total of 2 g/day. The dose used was much lower than the dose used for prophylaxis of bipolar disorder
(3) because we had been informed of successful clinical use of the lower dose (personal communication from D. Horrobin of Laxdale Ltd.). Hamilton depression ratings were done at baseline and weekly thereafter by an experienced psychiatrist who was blind to the treatment (B.N.). One patient in the placebo group dropped out by week 3 because her depressive symptoms got worse.
Results
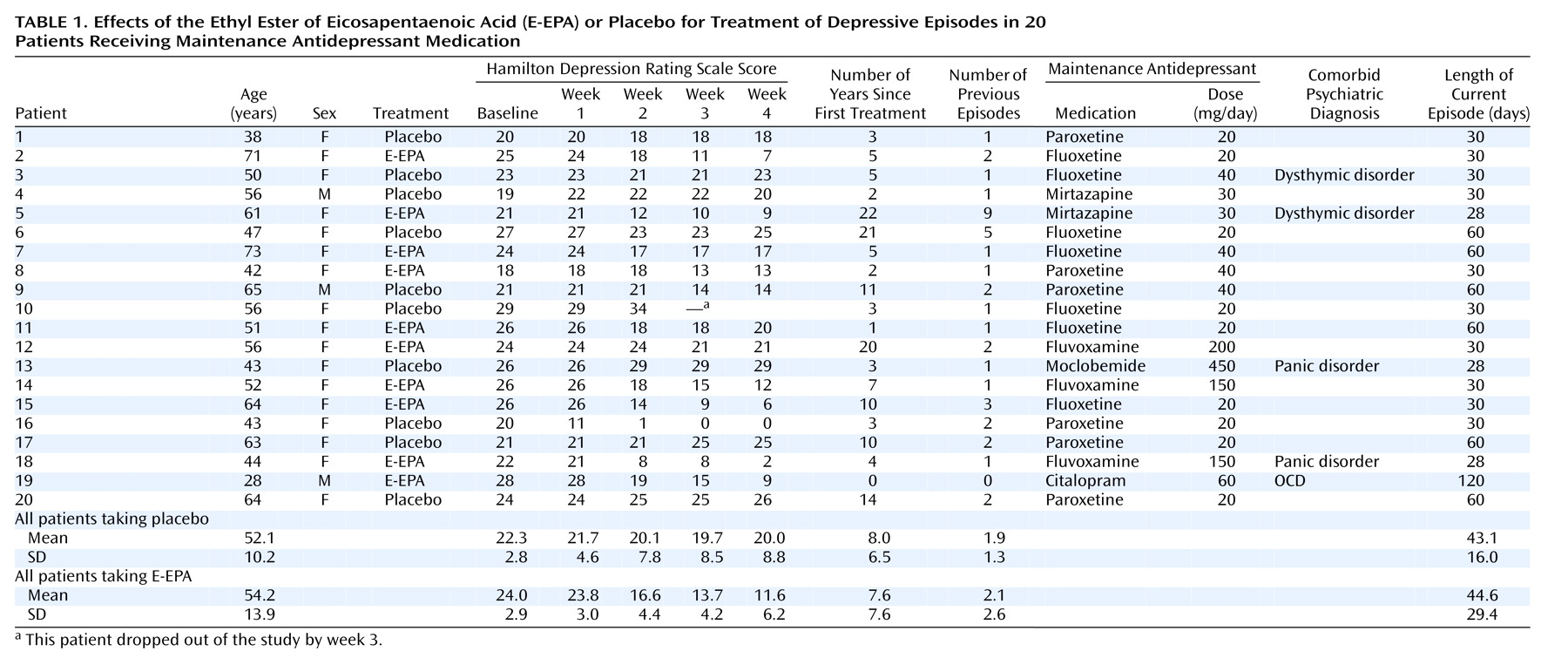
Table 1 illustrates the results of the study. Twenty patients participated. Seventeen were women, and three were men. Their mean age was 53.4 years (range=28–73). Two-way multivariate analysis of covariance of treatment by time (Greenhouse-Geisser corrected) with covariance for baseline was performed, with the last value carried forward for the patient who dropped out by week 3. Treatment and time showed a statistically significant interaction (F=17.6, df=1.6, 28.6, p<0.001). E-EPA was significantly different from placebo at week 2 (Newman-Keuls post hoc test, p<0.001), week 3 (Newman-Keuls post hoc test, p<0.001), and week 4 (Newman-Keuls post hoc test, p<0.001). Excluding the patient who dropped out of the study, we still found a statistically significant interaction between treatment and time (F=12.09, df=1.6, 27.8, p<0.001). The mean reduction of Hamilton depression scale score in patients receiving E-EPA was 12.4 points, compared with 1.6 in patients receiving placebo. This reduction was clinically meaningful: six of 10 patients receiving E-EPA but only one of 10 patients receiving placebo achieved a 50% reduction in Hamilton depression score.
No clinically relevant side effects were reported. No patient reported fishy sensations when asked specifically, and debriefing of the blind revealed a completely random guess rate by patient and clinician (B.N.).
Discussion
The present study is one of the first therapeutic trials of omega-3 fatty acids in unipolar depressive disorder
(9). The present study used E-EPA, and future studies will be necessary to compare the effectiveness of the different fatty acids in the treatment of depressive disorder, as well as the relevant dose-response curves.
The effect of E-EPA was significant from week 2 of treatment, similar to the time course for effectiveness of antidepressant medications. Item analysis showed that E-EPA had an effect on core depressive symptoms such as depressed mood, guilt feelings, and worthlessness as well as insomnia.
The present study illustrates the increasingly recognized fact that the composition of any group of patients included in a study is determined not only by formal inclusion criteria but by referral patterns, which depend on clinical setting and the perception of the trial by the network of referring clinicians. We found that patients referred for our trial could not be defined accurately merely by the entrance criterion of major depressive disorder. Although they were depressed despite receiving adequate current doses of antidepressant medication, the patients in the present study did not meet clinical criteria for treatment-resistant depressive disorder. All but one had been successfully treated with antidepressants in the past, had continued to take antidepressants because of relapse on cessation or dose lowering, but were not improving during the current episode despite clinical maneuvers such as dose increase, exhortation to greater compliance, or increased visit frequency with supportive psychotherapy. In retrospect, we described our clinical subjects as experiencing “breakthrough depression.”
The very low improvement rate in the placebo group is noteworthy and implies a degree of resistance to standard antidepressant therapy. It is thus not possible to distinguish whether E-EPA augments antidepressant action in the manner of lithium or has independent antidepressant properties of its own. Given the possible effects of omega-3 fatty acids on second messenger systems
(10), a lithium-like effect would be most interesting to explore.
The present study in unipolar depressive disorder is highly preliminary and requires replication in a larger sample, in a monotherapy design, and with a broad range of depression subtypes.