Most olanzapine-induced weight gain occurs early in treatment. Previously untreated patients seem to be particularly vulnerable to this side effect
(1). Olanzapine’s antagonistic activity at the serotonin-2C (5-HT
2C) receptor may account for its high propensity to cause weight gain
(2). Fluoxetine induces stimulation of the 5-HT
2C receptor, and its short-term administration has been associated with weight loss and an antibulimic effect
(3).
We examined whether fluoxetine coadministration attenuates olanzapine-induced weight gain in patients with first-episode schizophrenia.
Method
We studied 30 first-episode patients with DSM-IV schizophrenia (21 men, nine women) hospitalized at Tirat Carmel Mental Health Center (Israel). Diagnosis was based on the Structured Clinical Interview for DSM-IV. Participants had less than 4 weeks of antipsychotic drug exposure. We excluded uncooperative, aggressive, and suicidal patients and subjects with medical illnesses that could affect body weight. The results of physical examinations and laboratory tests were normal. The study was approved by the institutional review board. Written informed consent was obtained from all patients after they received a full explanation of study procedures.
The patients were randomly assigned to 8 weeks of olanzapine (10 mg/day at 8:00 p.m.) with fluoxetine (20 mg/day at 8:00 a.m.) (N=15) or olanzapine with placebo (at 8:00 a.m.) (N=15). Only trihexyphenidyl for extrapyramidal symptoms or lorazepam for insomnia or agitation was allowed as needed. The patients were not involved in weight reduction diets or programs.
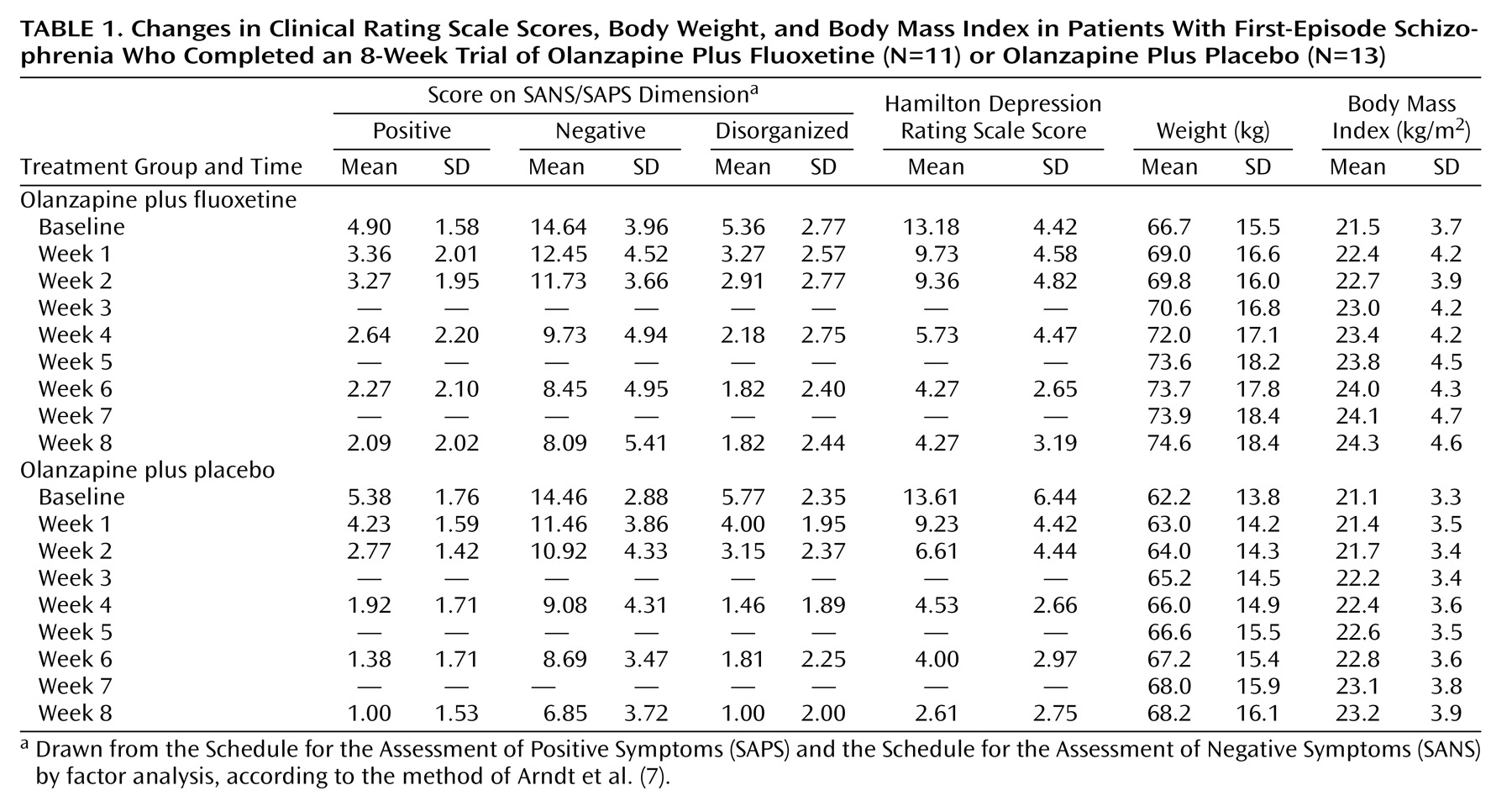
Weight and body mass index were measured at baseline and weekly at 8:00 a.m. before breakfast. Ratings with psychometric instruments were completed at baseline and at weeks 1, 2, 4, 6, and 8 and included the Scale for the Assessment of Positive Symptoms (SAPS)
(4), Scale for the Assessment of Negative Symptoms (SANS)
(5), and Hamilton Depression Rating Scale
(6). Three dimensions (positive, negative, and disorganized) were drawn from the SAPS and SANS by factor analysis, according to the method of Arndt et al.
(7).
Student’s t test, chi-square analysis, and Pearson’s correlation were used as appropriate. Weight, body mass index, and clinical rating scale scores were analyzed for the study completers by using analysis of covariance (ANCOVA) with repeated measurements, with corresponding baseline values as covariates. Intent-to-treat analysis was performed separately.
Results
Six patients withdrew from the study within the first 4 weeks because of lack of response (two patients receiving fluoxetine) and psychotic exacerbation (two fluoxetine patients and two placebo patients). The 11 completers in the fluoxetine group comprised nine men and two women with a mean age of 24.3 years (SD=4.4) and a mean duration of illness of 26.1 months (SD=55.4). The 13 completers in the placebo group included nine men and four women, who had a mean age of 26.1 (SD=7.9) and a mean duration of illness of 26.7 months (SD=28.2). The body mass indexes were within the normal range (18.5–24.9 kg/m2).
ANCOVA with repeated measurements revealed significant effects of time on the ratings for the SAPS/SANS dimensions (positive: F=29.25, df=4, 88, p<0.001; negative: F=26.71, df=4, 88, p<0.001; disorganized: F=29.49, df=4, 88, p<0.001) and on the Hamilton depression scale score (F=23.21, df=4, 88, p<0.001) (
Table 1). The patients receiving olanzapine plus placebo showed significantly greater reductions in scores on the positive and disorganized subscales than the patients receiving olanzapine plus fluoxetine (group-by-time interaction: F=5.23, df=4, 88, p=0.001, and F=3.43, df=4, 88, p=0.02, respectively). No such interaction was detected for the negative subscale (F=0.61, df=4, 88, p=0.66) or Hamilton depression scale (F=0.85, df=4, 88, p=0.50).
The two groups demonstrated similar gradual increases in body weight over the study period. Only two participants, both in the placebo group, did not gain weight. The ANCOVA with repeated measurements for weight and body mass index revealed significant time effects (F=28.90 and F=28.58, respectively, df=7, 120, p<0.001, for both) and a lack of a group effect (weight: F=1.23, df=1, 21, p=0.28; body mass index: F=2.02, df=1, 21, p=0.17) or group-by-time interaction (weight: F=0.43, df=7, 120, p=0.88; body mass index: F=0.22, df=7, 120, p=0.98) (
Table 1).
The mean weight gain at week 8 was 7.9 kg (SD=4.6) in the group receiving olanzapine plus fluoxetine group and 6.0 kg (SD=4.2) in the group receiving olanzapine plus placebo, a difference of 1.9 kg (t=0.78, df=22, p=0.44). According to power analysis for our group sizes and standard deviations, we would have detected with powers of 60% and 80% only differences in means larger than 4.17 kg and 5.25 kg, respectively. Nine patients in each group gained at least 7% of their initial weight, the cutoff for clinically relevant weight gain (χ2=0.50, df=1, p=0.48, n.s.).
There was no significant correlation between weight gain at 8 weeks and initial body mass index for either the fluoxetine group (r=0.54, df=9, p=0.09) or the placebo group (r=0.31, df=11, p=0.29). There was also no significant correlation between 8-week weight gain and change in score on the positive, negative, or disorganized subscale for either the fluoxetine group (r=0.08, r=0.21, r=–0.11, respectively, df=9) or the placebo group (r=–0.45, r=–0.17, r=–0.20, respectively, df=11).
Intent-to-treat analysis (N=15 in each group) yielded a mean weight gain of 6.1 kg (SD=5.3) in the fluoxetine group and 5.9 kg (SD=4.6) in the placebo group (t=0.11, df=28, p=0.91).
Discussion
We found that in first-episode schizophrenia patients, olanzapine treatment (coadministered with either placebo or fluoxetine) was associated with substantial weight gain. In addition, there was a significant between-group difference, in favor of the group receiving olanzapine plus placebo, in the reduction of positive and disorganized symptoms, suggesting that adding fluoxetine to olanzapine for first-episode schizophrenia patients may attenuate olanzapine’s antipsychotic effect.
The pattern of persistent and gradual increase in body weight in all patients except two from the placebo group indicates that fluoxetine coadministration is ineffective for preventing weight gain in olanzapine-treated patients with first-episode schizophrenia. In contrast to Gupta et al.
(8), we found no significant correlation between clinical response and weight gain. This discrepancy may be explained by differences in groups, design, and olanzapine dose. Basson et al.
(9) reported that low baseline body mass index, younger age, and increased appetite were the most significant predictors of weight gain associated with olanzapine treatment. We found no significant correlation between initial body mass index and change in body weight at the end of 8 weeks. The small group size and coadministration of fluoxetine may account, at least in part, for this discrepancy.
Several factors could account for the failure of fluoxetine to prevent weight gain in our patients. Fluoxetine’s stimulation of 5-HT may be insufficient to counteract the marked blockade of 5-HT
2C receptors exerted by olanzapine
(2). Histamine H
1 receptor antagonism may be involved in olanzapine-induced weight gain. Finally, a combination of 5-HT and norepinephrine reuptake inhibition, as exerted by the appetite suppressant sibutramine
(10), may be required for prevention of weight gain.