Schizophrenia is a life-shortening illness, with mortality rates among schizophrenic patients twice as high as in the general population
(1). Unnatural causes such as accidents and suicide account for only a portion of the increased mortality, and more than two-thirds of the variance has been explained by “natural causes,” including a variety of physical illnesses
(2). Life expectancy among schizophrenic patients is 20% shorter than in the general population, with circulatory, respiratory, and gastrointestinal illnesses accounting in part for this finding
(3). In addition, patients with schizophrenia also appear to have higher rates of impaired glucose tolerance, insulin resistance, and type II diabetes mellitus than the general population
(4).
However, most of the evidence indicating that type II diabetes mellitus occurs more commonly in schizophrenia has come from studies in which patients were either receiving neuroleptics or had been exposed to neuroleptics in the past
(5–
7). It is difficult to determine whether schizophrenia per se has an independent role in the development of abnormal glucose metabolism, as both conventional and atypical neuroleptics have been implicated in the pathogenesis of type II diabetes mellitus and impaired glucose tolerance
(8–
10). An independent role for the disease was suggested by the intriguing observations of investigators in the preneuroleptic era, although these data must be interpreted with caution because the definitions of diabetes and schizophrenia used then differ somewhat from those used today
(11–
14). Despite these reservations, support for the hypothesis that schizophrenia and diabetes may be linked independently of medication comes from the observation that the rate of type II diabetes mellitus in family members of schizophrenic patients is between 18% and 30%
(15), which is far higher than the rate in the population at large (1.2%–6.3%)
(16). Therefore, patients with schizophrenia and their first-degree relatives appear to be predisposed to developing type II diabetes mellitus. The objective of this study was to determine whether drug-naive, first-episode patients with schizophrenia have a higher frequency of impaired fasting glucose tolerance or of type II diabetes mellitus than a healthy volunteer group matched for age, sex, diet and exercise variables, and various anthropometric measures.
Method
Twenty-six Caucasian subjects (15 men and 11 women; mean age=33.6 years, SD=13.5) who fulfilled DSM-IV criteria for schizophrenia were recruited in this cross-sectional study. All patients were hospitalized and lived in the catchment area served by the hospital. They were referred to the study either by their family doctors or by the local emergency room staff. The study was approved by the hospital ethics committee. After a complete description of the study to the subjects, written informed consent was obtained. All patients were experiencing their first episode of schizophrenia and were drug naive. Their status was confirmed by collateral histories obtained from family members and the patients’ primary care physicians. None of the patients met the study exclusion criterion of a comorbid DSM-IV diagnosis, including alcohol or illicit drug abuse, and all were physically healthy. The patients were assessed for independent risk factors for type II diabetes mellitus, i.e., family history of type II diabetes mellitus, generalized obesity as indicated by body mass index, and abdominal obesity as indicated by waist circumference and waist-to-hip ratio. Although type II diabetes mellitus was not an exclusion criterion, none of the patients had a personal or family history of the disorder. The patients received a physical examination, a urine test for drug screening, and routine blood tests; all findings were within normal limits.
Illness-related variables were rated by the first author (M.C.M.R.) with the Brief Psychiatric Rating Scale (BPRS)
(17), Schedule for the Assessment of Negative Symptoms (SANS)
(18), and the Abnormal Involuntary Movement Scale (AIMS)
(19). The mean BPRS (normalized) and SANS scores were 54.8 (SD=6.6) and 37.5 (SD=17.5), respectively. The AIMS was used to ensure that patients with tardive dyskinesia did not enter the study; and the mean score was 0.0.
The normal comparison group, consisting of 26 Caucasian subjects (15 men and 11 women; mean age=34.4 years, SD=1.9), were physically healthy and had no personal or family history of psychiatric illness or type II diabetes mellitus. The comparison subjects were recruited from within the hospital, the affiliated university, and the general community. None of the comparison subjects was taking any form of prescribed or over-the-counter medication. The patients and normal comparison subjects were matched in terms of age, exercise and diet habits, smoking habits, and alcohol intake. The life-style factors of diet and exercise were rated by using the Dietary Instrument for Nutrition Education questionnaire
(20) and the Leisure Time Questionnaire
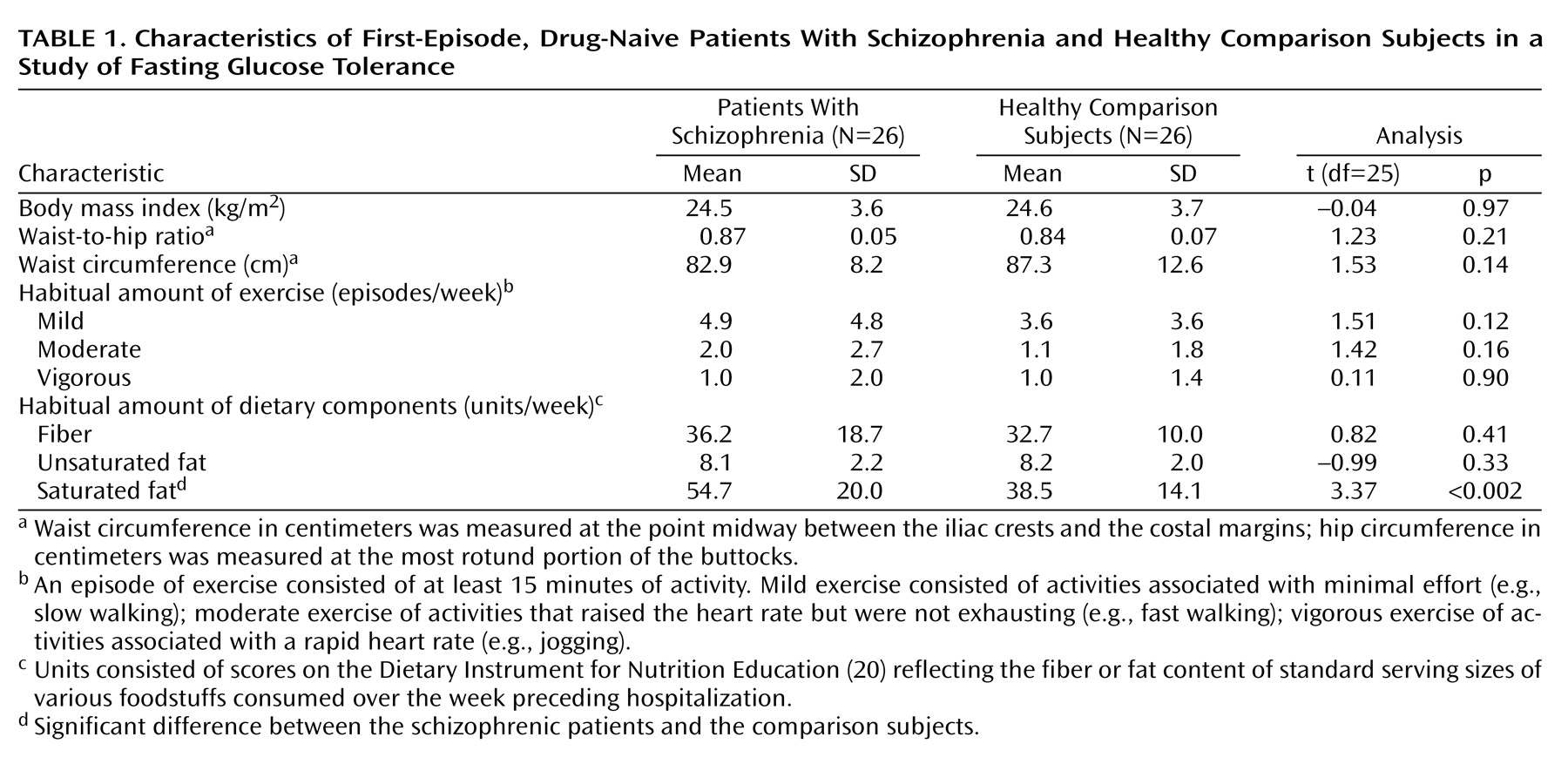
(21), respectively. Dietary variables were measured in units/week, consisting of scores on the Dietary Instrument for Nutrition Education reflecting the fiber or fat content of standard serving sizes of various foodstuffs consumed over the week preceding hospitalization. The patients did not differ from the comparison subjects in the usual amount of exercise or their intake of fiber or unsaturated fat; however, the patients consumed more saturated fat than did the comparison subjects (
Table 1).
Weight (kg), height (m), waist (cm), and hip (cm) measurements were taken for all subjects, and the waist-to-hip ratio and body mass index (kg/m2) were calculated for each subject. For the waist-to-hip ratio, the waist measurement was taken at the point midway between the iliac crests and the costal margins, and the hip measurement was taken at the most rotund portion of the buttocks.
Blood samples were taken from all subjects at 8:00 a.m. after a 12-hour overnight fast. Blood was withdrawn from an antecubital vein into Vacutainer tubes (Monovette, Sarstedt, Leicester, U.K.) for measurement of plasma levels of glucose, insulin, cortisol, and lipids. After centrifugation of blood at 2500
g for 10 minutes at 4°C, two aliquots of 2 ml each were placed in cryogenic tubes and frozen at –20°C until analysis. Serum glucose measures (mg/dl) were determined enzymatically by using the hexokinase method (Roche Diagnostics GmbH, Mannheim, Germany). Insulin concentrations were determined by using a commercial assay based on the microparticle enzyme assay (MEIA) technology (Dainbot, Tokyo, Japan). Plasma cortisol levels were determined by using fluorescence polarization immunoassay (Abbott Laboratories, Abbott Park, Ill.). Total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels were analyzed enzymatically by using commercial kits (Roche Diagnostics GmbH, Mannheim, Germany). The level of low-density lipoprotein (LDL) cholesterol was calculated by using the formula of Friedwald et al.
(22).
Impaired fasting glucose tolerance was defined by using the American Diabetic Association
(23) criteria as a plasma glucose level ≥110 mg/dl and ≤125 mg/dl. Insulin resistance was calculated with homeostasis model assessment
(24) by multiplying the fasting insulin level (mM/liter) by the fasting glucose level (mU/liter) and dividing the product by 22.5. Homeostasis model assessment is a mathematical method that has been compared with independent estimates of beta-cell function and insulin resistance.
Student’s t tests (two-tailed), chi-square tests, and Pearson’s product-moment correlation analyses were used, as appropriate, to compare results for the patients and the healthy subjects. All results are expressed as means and standard deviations. The data were analyzed by using Statgraphics, version 7.0
(25).
Results
Analysis of anthropometric and life-style data (
Table 1) showed no significant differences between the patient and comparison groups, except that the patients had higher saturated fat intake than the comparison subjects (mean=54.7 units/week, SD=20.0, versus mean=38.5 units/week, SD=14.1) (t=3.37, df=25, p<0.002). In contrast, the frequency of impaired fasting glucose tolerance, as defined by the American Diabetic Association
(23) criteria (>110 mg/dl and <126 μg/dl), was 15.4% (N=4) in the patient group and 0% in the comparison group, (χ
2=4.33, df=1, p<0.02). Compared with the healthy subjects, patients with schizophrenia had a significantly higher mean fasting plasma levels of glucose (mean=95.8 mg/dl, SD=16.9, versus mean=88.2 μg/dl for the comparison subjects, SD=5.4) (t=2.17, df=25, p<0.03), insulin (mean=9.8 μu/ml, SD=3.9, versus mean=7.7 μu/ml, SD=3.7) (t=2.05, df=25, p< 0.05), and cortisol (mean=499.4 nmol/liter, SD=161.4, versus mean=303.2 nmol/liter, SD=10.5) (t=5.11, df=25, p<0.0001). Insulin resistance, measured with homeostasis model assessment, was higher in the patients than in the comparison subjects (mean=2.3, SD=1.0, versus mean=1.7, SD=0.7) (t=2.56, df=25, p<0.01). Compared with the healthy subjects, the schizophrenic patients had significantly lower fasting plasma levels of cholesterol (mean=4.02 mmol/liter, SD=0.78, versus mean=4.57 mmol/liter for the comparison subjects, SD=0.81) (t=–2.52, df=25, p<0.02) and LDL cholesterol (mean=2.39 mmol/liter, SD=0.84, versus mean=2.91 mmol/liter, SD=0.69) (t=–2.47, df=25, p<0.02). However, no significant differences between groups were found in plasma levels of triglycerides (mean=0.99 nmol/liter, SD=0.43, for the patients versus mean=0.92 nmol/liter, SD=0.30, for the comparison subjects) (t=0.66, df=25, p<0.51) and HDL cholesterol (mean=1.20 mmol/liter, SD=0.44, versus mean=1.25 mmol/liter, SD=0.25) (t=–0.48, df=25, p<0.64).
In the patients with schizophrenia, fasting concentrations of plasma glucose correlated positively with waist-to-hip ratio (r=0.38, df=24, p<0.05), waist circumference (r=0.57, df=24, p<0.002), body mass index (r=0.39, df=24, p<0.05), plasma triglyceride level (r=0.49, df=24, p<0.01), and age (r=0.61, df=24, p<0.001), although not with severity of illness as measured with the BPRS (r=–0.22, df=24, p<0.28) or the SANS (r=0.12, df=24, p<0.57). There was also a positive correlation between waist-to-hip ratio and plasma triglyceride level (r=0.45, df=24, p<0.02) and a negative correlation between waist-to-hip ratio and HDL cholesterol (r=–0.58, df=24, p<0.002).
Discussion
To our knowledge, this is the first study in the postneuroleptic era to show a higher rate of impaired fasting glucose tolerance, as defined by American Diabetic Association criteria
(23), in first-episode, drug-naive patients with DSM-IV schizophrenia than in matched comparison subjects. In this study, more than 15% of the patients with schizophrenia had impaired fasting glucose tolerance, compared to none of the matched healthy comparison subjects. In addition, the patients had higher fasting plasma levels of glucose, insulin, and cortisol and were more insulin resistant than the healthy comparison subjects. An unexpected finding was that although the patients’ diets were higher in saturated fat, they had lower fasting plasma levels of total cholesterol and LDL cholesterol than the normal comparison subjects. Last, in patients with schizophrenia, fasting concentration of plasma glucose correlated positively with waist-to-hip ratio, waist circumference, body mass index, plasma triglyceride level, and age. There was also a positive correlation between waist-to-hip ratio and plasma triglyceride level and a negative correlation between waist-to-hip ratio and HDL cholesterol level.
The 15.4% frequency of impaired fasting glucose tolerance observed in the patients in this study, who had a mean age of 33.6 years, is higher than the age-adjusted (35–64 years) frequencies of 11.8% in men and 5.2% in women in a recent study of the general population in three regions in France
(26). From a prognostic perspective, earlier studies have suggested that one-third of those with hyperinsulinemia, insulin resistance, and impaired fasting glucose tolerance will eventually develop type II diabetes mellitus and macrovascular disease
(27–
32). Thus, schizophrenic patients with impaired fasting glucose tolerance may be predisposed to developing type II diabetes and various cardiac arrhythmias, and the high frequency of impaired fasting glucose tolerance in this group may explain in part the reduced life expectancy of patients with schizophrenia.
If a significant proportion of persons with impaired fasting glucose tolerance go onto develop type II diabetes, it would seem logical that the pathogenetic mechanisms underlying both conditions would be similar
(33). With this in mind, it is prudent to consider why the patients with schizophrenia in this study appear prone to develop this precursor of type II diabetes. One potential reason may be that patients did not fast before being tested. However, all subjects with schizophrenia in our study were hospitalized, and fasting was strictly enforced for the 10–12 hours before plasma sampling.
Medication may induce hyperglycemia and hyperinsulinemia
(5–
7,
34). This is an unlikely explanation for our findings, as the patients in this study were drug naive and were experiencing their first episode of schizophrenia; the latter was corroborated by collateral histories obtained from family members and primary care physicians. Further support for the hypothesis that problems with abnormal glucose metabolism may occur in schizophrenia independently of antipsychotic exposure comes from a study by Mukherjee et al.
(35). In their study, 15.8% of the patients with schizophrenia were hyperglycemic (on the basis of two measurements of fasting plasma glucose levels), but those who were taking no medication had higher rates of hyperglycemia than those who were taking haloperidol. In addition, Mukherjee et al. showed that the prevalence of type II diabetes in patients with schizophrenia was age dependent, with rates of 0% in those below age 50 years, 12.9% in those aged 50–59 years, and 18.9% in those aged 60–69 years. This increase in rates mirrors that found in the general population, in which the rates of type II diabetes are approximately 1.2% in those aged 18–45 years and 6.3% in those aged 46–64 years
(16). Yet, the average age of the patients in our study was 33.6 years (SD=13.5), and thus it is highly unlikely that their age was a factor in their high rates of impaired fasting glucose tolerance.
Leaving aside the issues of medication and age, factors such as ethnicity, physical inactivity, and smoking and diet habits may also play a crucial role in the development of type II diabetes
(36,
37). However, we ensured that patients and comparison subjects were matched in terms of smoking and physical exercise habits, so these two parameters cannot explain the higher rates of impaired fasting glucose tolerance and insulin resistance in the schizophrenic patients. Both the schizophrenic patients and the comparison subjects were from the same homogenous ethnic background. As for diet, the patients and the healthy comparison subjects ate similar amounts of fiber and unsaturated fats, but the schizophrenic subjects consumed larger amounts of saturated fat. Two cross-sectional studies and a small prospective study have suggested a positive association between a high intake of saturated fat and hyperglycemia
(38–
40), although several larger cohort studies in which type II diabetes was the endpoint did not support this observation
(41–
43). Indeed, a high intake of saturated fat would increase LDL and HDL cholesterol content and reduce triglyceride levels. These lipid patterns were not seen in the patients in our study, and, therefore, we do not believe that the higher level of dietary saturated fat in the patients can explain the findings. In addition, hypercholesterolemia has been associated with poor glycemic control; however, the patients in our study had lower levels of cholesterol than the normal comparison subjects
(44). Last, some researchers have contended that patients with schizophrenia who are severely ill with either negative or positive symptoms may have poorer glycemic control
(34,
45). Yet our finding of no correlation between fasting glucose levels and severity of illness as measured by either the SANS or BPRS would argue strongly against this explanation.
Blood glucose levels have also been reported to be higher in patients with tardive dyskinesia
(46). Indeed, some evidence suggests that hyperglycemia associated with insulin resistance may contribute to the pathogenesis of tardive dyskinesia
(47–
49). For this reason, we looked for involuntary movements in the drug-naive schizophrenic subjects by using the AIMS. No patients, however, showed evidence of involuntary movements. As the patients who were recruited to the study had no history of exposure to antipsychotic medication and were recruited on their first presentation with psychosis, the lack of involuntary movements is not surprising.
Obesity is a well-recognized risk factor for the development of diabetes
(50). However, both the patients and the comparison subjects in our study were matched in terms of body mass index, waist circumference, and waist-to-hip ratio and were not obese. These findings are in keeping with the observation that although a minority of patients with schizophrenia are obese, the overall rates of obesity in that patient group do not differ from that in the general population
(51). Yet, obesity is a heterogenous condition in that some individuals with large amounts of body fat have few metabolic complications, while others who are lean or are minimally overweight may develop type II diabetes and cardiovascular diseases
(52–
54). Therefore, it is open to question whether obesity is an independent risk factor for developing these metabolic complications. Indeed, there appears to be a subgroup of patients who have excess intraabdominal (visceral) fat and are greatly predisposed to the development of type II diabetes, dyslipidemia, and certain cardiovascular illnesses
(55,
56). Increased visceral fat, with or without obesity, is associated with hyperinsulinemia, insulin resistance, and glucose intolerance, all of which are precursors to type II diabetes
(57). Yet, the patients with schizophrenia in our study had higher levels of plasma insulin and were more insulin resistant than the comparison subjects, and neither group were obese, which raises the issue of whether patients with schizophrenia have higher rates of visceral obesity. We believe that they do, as drug-naive, first-episode patients with schizophrenia have been shown to have 3.4 times more intraabdominal fat than comparison subjects, which may in turn explain why they are at a higher risk for various metabolic complications than comparison subjects who do not have excessive abdominal fat
(58). Some of these features—visceral obesity, dyslipidemia, impaired glucose tolerance/type II diabetes, hyperinsulinemia/insulin resistance, and cardiovascular disease—constitute the metabolic syndrome
(59).
This study also demonstrated positive correlations between indirect measures of visceral obesity, such as waist-to-hip ratio and waist circumference, and plasma levels of glucose and triglycerides, adding further credence to the hypothesis that schizophrenia is associated with the metabolic syndrome
(4). Although no comprehensive explanation has been put forward to account for the co-occurrence of this group of conditions, it would appear that dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis may play a role
(60). Indeed, conditions such as Cushing’s syndrome and melancholic depression, which are associated with hypercortisolemia, are associated with both excessive amounts of visceral fat deposition and the development of various features of the metabolic syndrome
(61–
63). Schizophrenia is also associated with abnormalities of the HPA axis
(64–
67). Using a crude indicator of HPA axis activity in this study, we have shown that many schizophrenic patients had hypercortisolemia, which in turn may explain why they have excessive visceral fat, hyperglycemia, hyperinsulinemia, and insulin resistance. Alternatively, the stress of hospitalization may lead to impaired fasting glucose tolerance, as in major depression, and the endocrine abnormality may resolve with successful treatment
(68). This hypothesis may well explain our findings, yet arguing against it are two observations. First, the rates of type II diabetes mellitus in first-degree relatives of patients with schizophrenia exceeds that in the general population
(15). Second, HPA axis abnormalities are more likely to occur in family members of patients with schizophrenia than in those of normal comparison subjects
(67). These findings suggest that stress axis changes and problems with glucose metabolism are associated with the illness rather than with factors relating to the immediate environment.
In conclusion, 15.4% of the drug-naive, first-episode patients with schizophrenia in our study had impaired fasting glucose tolerance, compared to none of the matched healthy subjects. The patients also had higher levels of plasma glucose, insulin, and cortisol and were less insulin sensitive than the comparison subjects. The findings of this and other studies suggest that the illness of schizophrenia is associated with various aspects of the metabolic syndrome, which in turn may explain why patients with this illness die prematurely.