Prazosin substantially reduced trauma-related nightmares and globally rated severity of posttraumatic stress disorder (PTSD) in open-label studies
(1–
3). Prazosin is a centrally active α
1 adrenergic antagonist long available for treating hypertension
(4) that should counteract in part the excessive brain noradrenergic activity reported in PTSD
(5).
Method
Ten male Vietnam combat veteran outpatients (mean age=53 years, SD=3) provided signed informed consent for participation in this study, which was approved by the University of Washington institutional review board. All of the patients met DSM-IV criteria for PTSD and had experienced PTSD symptoms since their return from Vietnam at least 25 years earlier. Five patients met criteria for alcohol abuse in the past, but all had been free of alcohol or other substance abuse for at least 6 months. All had frequent and severe combat-trauma-related nightmares, as defined by a score of 6 or higher on the Clinician-Administered PTSD Scale
(6) recurrent distressing dreams item (maximum score=8), despite trials of psychoactive medications. Nine were receiving disability compensation for PTSD. Seven were receiving one or more of the following medications for PTSD: selective serotonin reuptake inhibitors (N=5), trazodone (N=2), benzodiazepines (N=4), anticonvulsants (N=2), hydroxyzine (N=2), and risperidone (N=1). Medications and psychotherapy were maintained unchanged during the study.
Each subject participated in a 20-week, two-period, two-treatment (prazosin and placebo) crossover study. Characteristics of prazosin and of the study group satisfied criteria for a classic crossover design
(7). First, veterans responsive to open-label prazosin almost always returned to their pretreatment nightmare intensity 1 or 2 days after prazosin discontinuation (i.e., there was no “carryover” therapeutic effect). Second, PTSD symptoms in these chronic patients were generally stable.
Following behavioral ratings (baseline 1), the patients were randomly assigned to prazosin first (N=5) or placebo first (N=5). Three-week dose titration was followed by 6-week maintenance treatment at the maximum achieved dose. At the end of week 9 (endpoint 1), the patients entered a 2-week no-study-drug washout period. At the end of week 11 (baseline 2), the patients were “crossed over” to the other treatment condition. The second drug then was titrated upward for 3 weeks, followed by a 6-week maintenance treatment period terminated at week 20 (endpoint 2).
Capsules contained prazosin 1 mg or placebo. To avoid possible “first-dose” orthostatic syncope, prazosin should be initiated at a 1-mg dose and then increased
(8). One mg/day (or equivalent placebo) at bedtime for 3 days was followed by 2 mg/day for 4 days. If nightmares were not markedly improved and adverse effects were clinically acceptable (as determined by a research psychiatrist blind to treatment condition), the dose was increased to 4 mg/day at bedtime for 7 days. Using the same titration guidelines, we increased the dose to 6 mg/day at bedtime for 7 days and then to 6 mg/day at bedtime plus 4 mg/day at 3:00 p.m.
The Clinician-Administered PTSD Scale was completed at baselines and endpoints. The Clinical Global Impression (CGI) of change
(9) was administered at endpoints. For those few patients who did not complete a treatment phase (see Results), endpoint ratings were obtained at study discontinuation; these last observations were carried forward as imputed endpoints.
The Clinician-Administered PTSD Scale and CGI of change were administered by a research psychiatrist (E.D.K., E.R.P., or D.J.D.) or clinical psychologist (M.M.M. or K.S.-T.) blind to medication condition and blood pressure. These raters had established excellent interrater reliability on both instruments. Each subject had the same rater throughout. Primary outcome measures were the Clinician-Administered PTSD Scale recurrent distressing dreams item and difficulty falling or staying asleep item and the CGI of change for overall PTSD severity and functional status. Secondary outcome measures were Clinician-Administered PTSD Scale total score (17 symptom items) and three symptom cluster scores (reexperiencing/intrusion, avoidance/numbing, and hyperarousal). Differences between baseline and endpoint (change scores) for all outcome measures (except for CGI of change) were calculated for each treatment condition regardless of treatment order and compared between treatment conditions by two-tailed paired t test. Effect sizes were calculated as mean posttreatment prazosin score minus mean posttreatment placebo score divided by the standard deviation of the mean posttreatment placebo score
(10).
Results
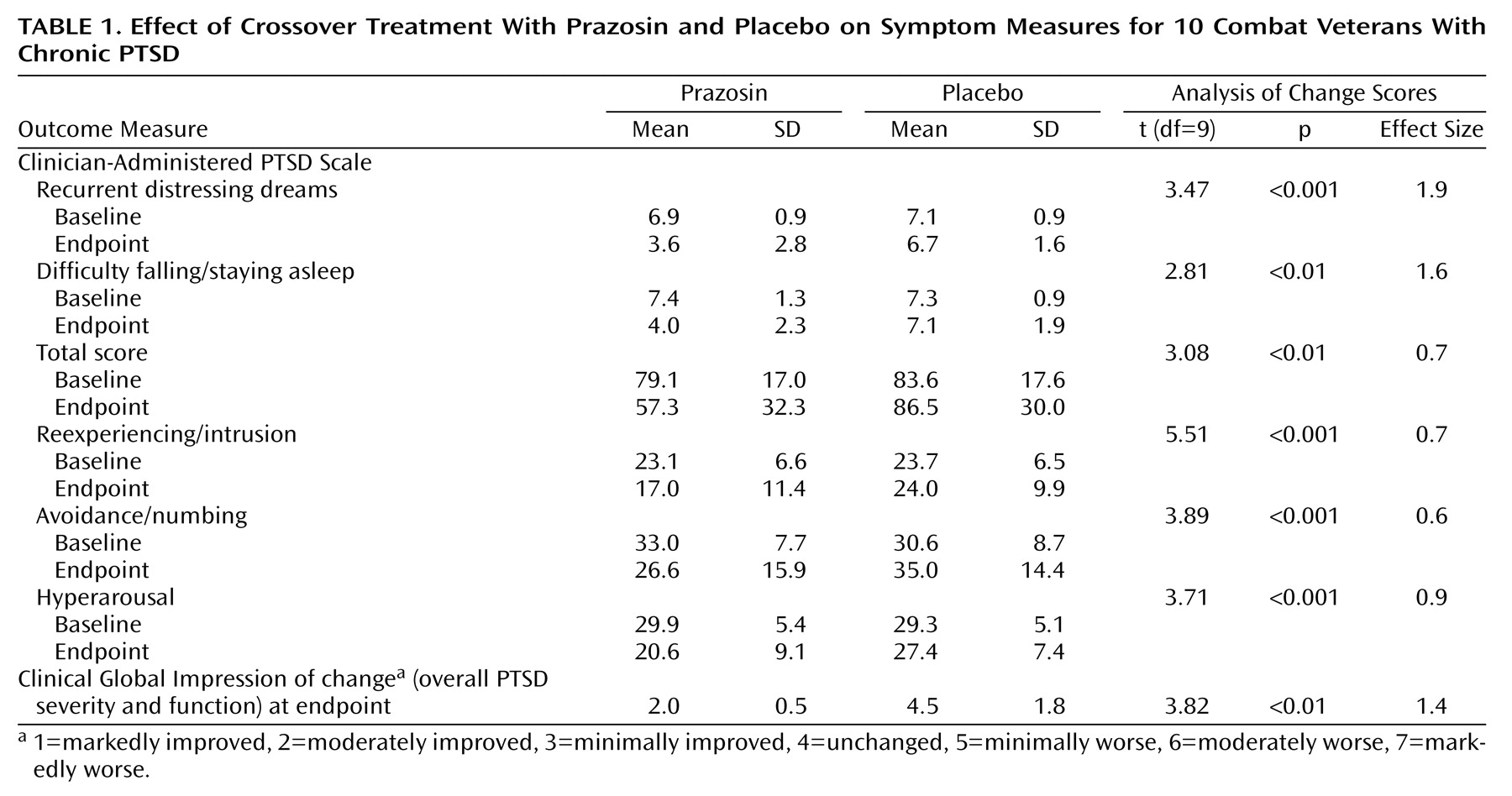
Subjects were more improved when they were taking prazosin than when they were taking placebo on the primary outcome measures of nightmares, sleep disturbance, and global change in PTSD severity and functional status (
Table 1). Moreover, prazosin was more effective for reexperiencing/intrusion, numbing/avoidance, and hyperarousal symptom cluster scores as well as total scores on the Clinician-Administered PTSD Scale. Effect size analyses for dependent variables showed robust and clinically meaningful reductions in symptoms across all outcomes measured
(10).
Prazosin was very well tolerated. Two patients experienced mild orthostatic systolic blood pressure decreases (10 to 20 mm Hg) and dizziness early during prazosin titration, which resolved as the dose was increased. At prazosin endpoint, mean systolic blood pressure was 135 mm Hg (SD=12) supine and 129 mm Hg (SD=12) standing; diastolic was 89 mm Hg (SD=8) supine and 84 mm Hg (SD=15) standing.
All patients completed all conditions except for those in the second placebo condition. Five patients experienced a rapid return of distressing nightmares during postprazosin washout. Four experienced no benefit from their second placebo treatment and insisted on discontinuing the study so they could be given open-label prazosin; these patients rapidly improved after receiving the active drug. Last observation carried forward analysis was selected to impute conservative endpoint 2 values for these subjects. Empirically, the change scores for these early termination patients were very similar to those for the five first-period placebo subjects.
Discussion
PTSD nightmares appear to arise from light sleep and/or disrupted REM sleep
(11). Prazosin reduces light sleep and normalizes REM sleep
(12). Prazosin reduces secretion of corticotropin-releasing hormone
(13), a neuropeptide elevated in PTSD
(14). The high CNS noradrenergic outflow in PTSD
(5) likely stimulates a
1 adrenergic regulation of the prefrontal cerebral cortex, disrupting cognitive processing and increasing fear responses
(15). This is corrected by prazosin
(15).
These results support the efficacy and safety of prazosin for trauma-related nightmares, sleep disturbance, and overall PTSD severity and function in previously treatment-resistant combat veterans. Prazosin offers a novel and inexpensive approach to nightmare reduction and other PTSD symptom relief for combat veterans. Further studies are necessary to replicate these findings and to determine if prazosin is effective in civilian trauma PTSD.