Until recently, pharmacologic treatment of generalized anxiety disorder consisted of the benzodiazepines, buspirone, and monoamine reuptake inhibitor antidepressants. Recently, management of generalized anxiety disorder has shifted from benzodiazepines toward antidepressants such as venlafaxine, driven by the recognition that 1) the antidepressants have antianxiety effects, 2) depressive symptoms that sometimes accompany generalized anxiety disorder may not respond well to benzodiazepines, and 3) benzodiazepines, despite their long record of safety, do carry some risk of abuse, dependence, and associated problems such as withdrawal effects. However, the delayed onset of clinical effect seen with antidepressant drugs and buspirone is a limitation and disadvantage compared with benzodiazepines, which are rapidly effective in many generalized anxiety disorder patients. For generalized anxiety disorder patients who do not have significant depressive symptoms
(1), an effective anxiolytic that is devoid of the liabilities of the benzodiazepines would provide these patients with an alternative treatment.
Recent attempts to develop nonbenzodiazepine anxiolytic agents with novel mechanisms have been mostly unsuccessful, as demonstrated by the poor outcome of trials of serotonin (5-HT)
3 antagonists
(2,
3) and CCK-B antagonists
(4–
6). Buspirone, although approved for generalized anxiety disorder treatment, has hardly supplanted benzodiazepines and has not shown efficacy in controlled trials of other anxiety disorders, such as social phobia
(7) and panic disorder
(8). One novel agent, pregabalin, a structural analogue of γ-aminobutyric acid (GABA), is currently in development as an anxiolytic on the basis of its profile of pharmacologic activity in animal behavioral models such as the Vogel conflict test
(9), commonly used to screen for antianxiety drugs. In early safety studies, pregabalin was rapidly absorbed, with a linear pharmacokinetic profile and a plasma half-life of about 6 hours
(10,
11). Pregabalin does not bind to plasma proteins and is excreted unchanged via the kidneys. Studies in healthy volunteers exposed to daily doses as high as 900 mg for 2–4 weeks have found no evidence of an abstinence syndrome following abrupt discontinuation of pregabalin
(10). On the basis of these highly desirable characteristics, we studied the efficacy and safety of pregabalin in the treatment of patients with generalized anxiety disorder.
Method
The study adhered to the principles of the Declaration of Helsinki, Good Clinical Practice Guidelines, and standard quality assurance procedures routinely used for Parke-Davis-sponsored clinical trials. The protocol was approved for each site by an institutional review board. Each patient received an explanation of the study before signing an instrument of written informed consent. Consent was obtained prior to any study-related activities.
The study was conducted at five outpatient clinical research sites based in Seattle; Portland, Ore.; Lansing, Mich.; Los Angeles; and Durham, N.C. The principal investigators were trained psychiatrists with extensive experience in conducting clinical trials in mood and anxiety disorders.
Subjects were outpatients 18 years of age or older, recruited through clinic referrals or from advertisements, provided they met a diagnosis of generalized anxiety disorder according to DSM-IV criteria. Patients were excluded if they suffered from any axis I disorder except dysthymia, simple phobia, social phobia, somatization disorder, or a history of major depressive disorder. Also, patients at suicide risk, as judged by the clinician on the basis of history or current severity of suicidal ideation, were excluded. Patients were required to be free of psychotropic medications for 2 weeks (5 weeks for fluoxetine) before enrollment. A urine drug screen was performed at screening and at termination, although a positive result at screening was not exclusionary. No psychotropic medications were allowed during the study with the exception of zolpidem (5 mg), which was permitted on an as-needed basis for extreme sleeplessness. Zolpidem was not to be taken for more than 2 nights per week and not to be taken the night before a clinic visit. Women of childbearing potential were required to be using contraception.
Assessments
Patients underwent a clinical assessment that included a diagnostic interview with a psychiatrist (either the principal investigator or a subinvestigator) and the administration of the Mini International Neuropsychiatric Interview
(12) to confirm the diagnosis of generalized anxiety disorder and to check for excluded axis I diagnoses. Divergent findings between the clinical interview and the Mini International Neuropsychiatric Interview were resolved by the judgment of the principal investigator. A medical and psychiatric history was obtained, and physical and laboratory examinations were carried out to ensure eligibility.
At the screening and treatment assignment visits, patients were required to have a Covi Anxiety Scale
(13) total score ≥9 and Raskin Depression Scale
(14) total score ≤7 to ensure that anxiety was the predominant presentation among patients with depressive symptoms. The severity of anxiety symptoms was assessed by the clinician-administered Hamilton Anxiety Rating Scale
(15) (score range=0–56), which was collected at each visit. Patients were required to have a Hamilton anxiety scale total score ≥20 at both the screening and treatment assignment visits. Depressive symptoms were measured by the 17-item Hamilton Depression Rating Scale
(16) (score range=0–52) at screening and termination. Patients with a score ≥2 on Hamilton depression scale item 3 (suicidal ideation) at the screening evaluation were excluded.
Global clinical assessment was conducted by using the Clinical Global Impression (CGI) change rating (1=very much improved, 7=very much worse). The Physician Withdrawal Checklist was used to assess symptoms commonly associated with benzodiazepine withdrawal during the taper phase. The Physician Withdrawal Checklist is a clinician-rated instrument that measures 20 common symptoms of withdrawal (score range=0–60)
(17).
Procedure
This was a double-blind, placebo-controlled comparison of pregabalin, 150 mg/day (50 mg t.i.d.); pregabalin, 600 mg/day (200 mg t.i.d.); and lorazepam, 6 mg/day (2 mg t.i.d.). The study had three phases: a 1-week placebo lead-in, a 4-week double-blind phase, and a 1-week taper. The 1-week, single-blind placebo lead-in phase was intended to establish the stability of generalized anxiety disorder symptoms and eliminate the effects of prior treatments. If patients still met study inclusion criteria at the end of the lead-in phase, as confirmed by a second clinical interview with the psychiatrist, they were randomly assigned to one of the four treatment conditions. Study medication was titrated during the first 6 days of double-blind treatment. On day 1, subjects received one-sixth of the randomly assigned dose, which was then increased daily until the targeted dose was reached. Patients were seen at weekly visits by the study site coordinator and the psychiatrist (either the principal investigator or a subinvestigator). Patients could be evaluated by different personnel on different visits but, as far as possible, the final efficacy and safety assessments were made by the same psychiatrist who did the baseline evaluations. Following 4 weeks of treatment, the final efficacy assessments were made (termination visit). Study medication dose was tapered over 1 week, and the follow-up visit was conducted. Adverse events that were reported spontaneously in response to a nondirected query by the investigator were collected at each visit along with vital signs. Physical examinations, clinical laboratory tests, and 12-lead ECGs were performed at screening and termination visits.
The Physician Withdrawal Checklist was administered at the termination and follow-up visits.
Guidelines for Early Patient Withdrawal
Every effort was made within the bounds of safety and patient choice to have patients complete the study. However, study medication could be discontinued at any time during the study after withdrawal of consent by the patient, at the investigator’s discretion if a patient developed a severe adverse reaction or a significant intercurrent illness, or if a patient missed more than seven consecutive doses of study medication. In the event of a patient’s early termination, all end-of-study efficacy and safety procedures were to be performed, a taper from study medication was to be initiated, and a follow-up visit was to be scheduled to assess symptoms of withdrawal following taper from study medication.
Statistical Analysis
The primary efficacy parameter was change from baseline to endpoint (week 4 or the last observation from the double-blind phase carried forward) in total score on the Hamilton anxiety scale. The study was powered to detect a mean difference in change in score on the Hamilton anxiety scale of 3.5 (SD=7.0) between pregabalin and placebo treatment groups. With 64 patients per treatment group, the study provided 80% power to detect a 3.5-point difference in change in score on the Hamilton anxiety scale between placebo and pregabalin, with two-sided testing for two treatment effects (pregabalin, 150 mg/day, versus placebo; pregabalin, 600 mg/day, versus placebo) and an experiment-wise alpha level of 0.05.
All statistical analyses were performed by using the SAS statistical package (version 6.12)
(18). Conclusions from hypothesis testing were based on two-sided p values. Hochberg’s adjustment for multiple comparisons
(19)(20) was used for the primary analysis. All other efficacy analyses were evaluated at alpha=0.05.
The primary efficacy parameter, baseline-to-endpoint change in Hamilton anxiety scale score, was analyzed by using an analysis of covariance (ANCOVA) model that included the effects of treatment and center, with baseline Hamilton anxiety scale total score entered as a covariate
(20). Adjusted (least squares) means and 95% confidence intervals were calculated, and Hochberg’s adjustment for multiple comparisons was used to test 1) the treatment effect of pregabalin, 150 mg/day, versus placebo; and 2) the treatment effect of pregabalin, 600 mg/day, versus placebo. Since an interim analysis was performed for administrative reasons at p=0.001, the final primary analysis was evaluated at the 0.049 level. Using Hochberg’s approach, we ranked the p values for these two comparisons from largest to smallest. If the larger of these two p values was statistically significant when evaluated at the 0.049 level, then both comparisons were declared statistically significant. If not, the smaller p value was evaluated at the 0.0245 level and, if statistically significant, only the comparison that yielded the smaller p value was declared statistically significant.
In order to assess the treatment effect over time, an ad hoc analysis of Hamilton anxiety scale scores at each weekly visit (observed cases) was performed. Weekly Hamilton anxiety scale change scores were analyzed by ANCOVA with a model that included the effects of treatment and center, with baseline Hamilton anxiety scale score entered as a covariate.
Secondary analyses were performed for the primary efficacy parameter, baseline-to-endpoint change in Hamilton anxiety scale score, by using ANCOVA models in the same manner as for the primary analysis except that all pairwise comparisons were performed and evaluated for significance at the 0.05 level. The comparisons were 1) pregabalin, 150 mg/day, versus pregabalin, 600 mg/day (dose-response relationship); 2) lorazepam versus placebo; 3) pregabalin, 150 mg/day, versus lorazepam; and 4) pregabalin, 600 mg/day, versus lorazepam. The effect of pregabalin treatment relative to placebo in terms of response (defined as a ≥50% decrease from baseline to endpoint in Hamilton anxiety scale total score and a CGI change rating of “much improved” or “very much improved” at endpoint) was evaluated by using logistic regression after we adjusted for center.
The adverse events recorded by investigators were mapped to preferred terms by using the COSTART dictionary
(21). Only treatment-emergent signs and symptoms were summarized. Each individual adverse event was counted only once, regardless of the number of times the patient experienced the event, by using the maximum intensity recorded.
Withdrawal Effects
Withdrawal was assessed by subtracting Physician Withdrawal Checklist scores at endpoint (week 4) from those at follow-up (week 5). The change scores were analyzed by using ANCOVA models in the same manner as for the primary analysis. The analyses included comparisons of the withdrawal effect of 1) pregabalin, 150 mg/day, versus placebo; 2) pregabalin, 600 mg/day, versus placebo; and 3) lorazepam versus placebo.
Results
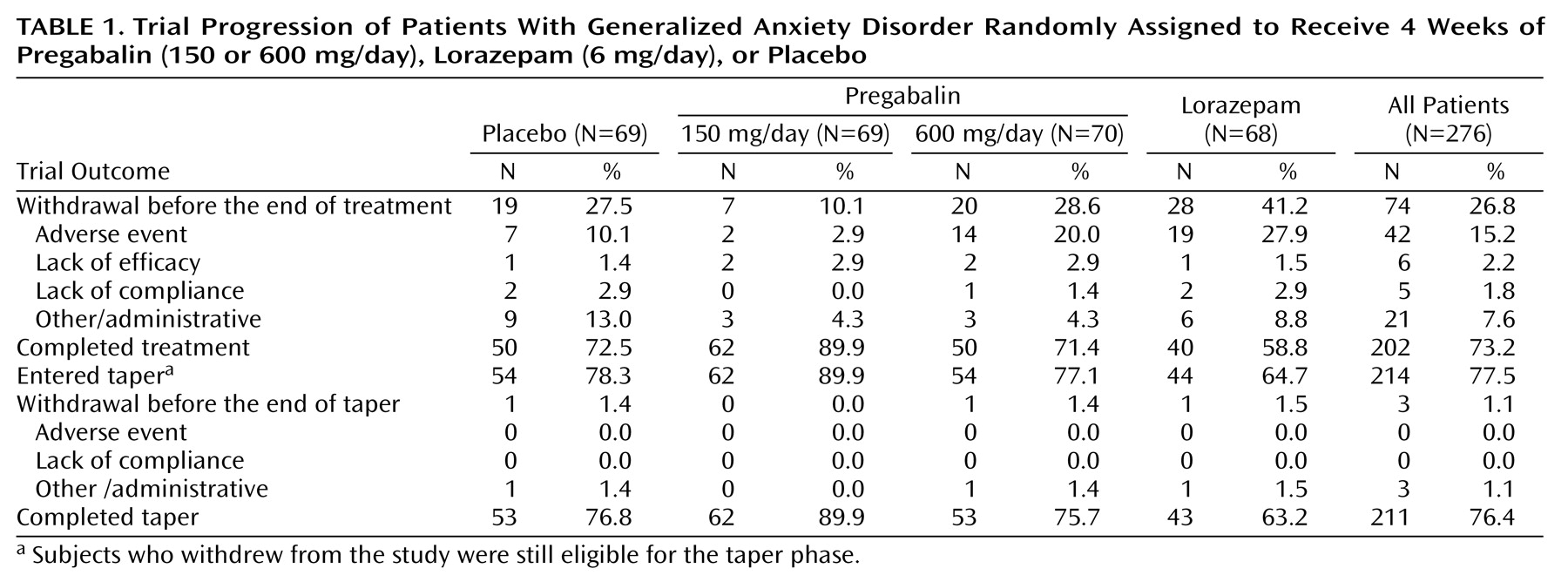
A total of 361 subjects were screened. Of these, 84 were excluded from the study because they did not meet inclusion criteria (N=31), experienced an adverse event (N=1), or because of other administrative reasons (N=52). Of the 277 patients randomly assigned to a treatment condition, 276 received at least one dose of double-blind study medication and were included in the intent-to-treat population (
Table 1). Some patients withdrew from the trial before completing the scheduled treatment period. The completion rate was lower for patients given lorazepam than for those given placebo or pregabalin (either 150 or 600 mg/day). Most early withdrawals during the double-blind treatment phase were due to adverse events. Early withdrawals due to adverse events were more frequent in the groups given lorazepam and pregabalin, 600 mg/day, than in those given placebo or pregabalin, 150 mg/day (
Table 1).
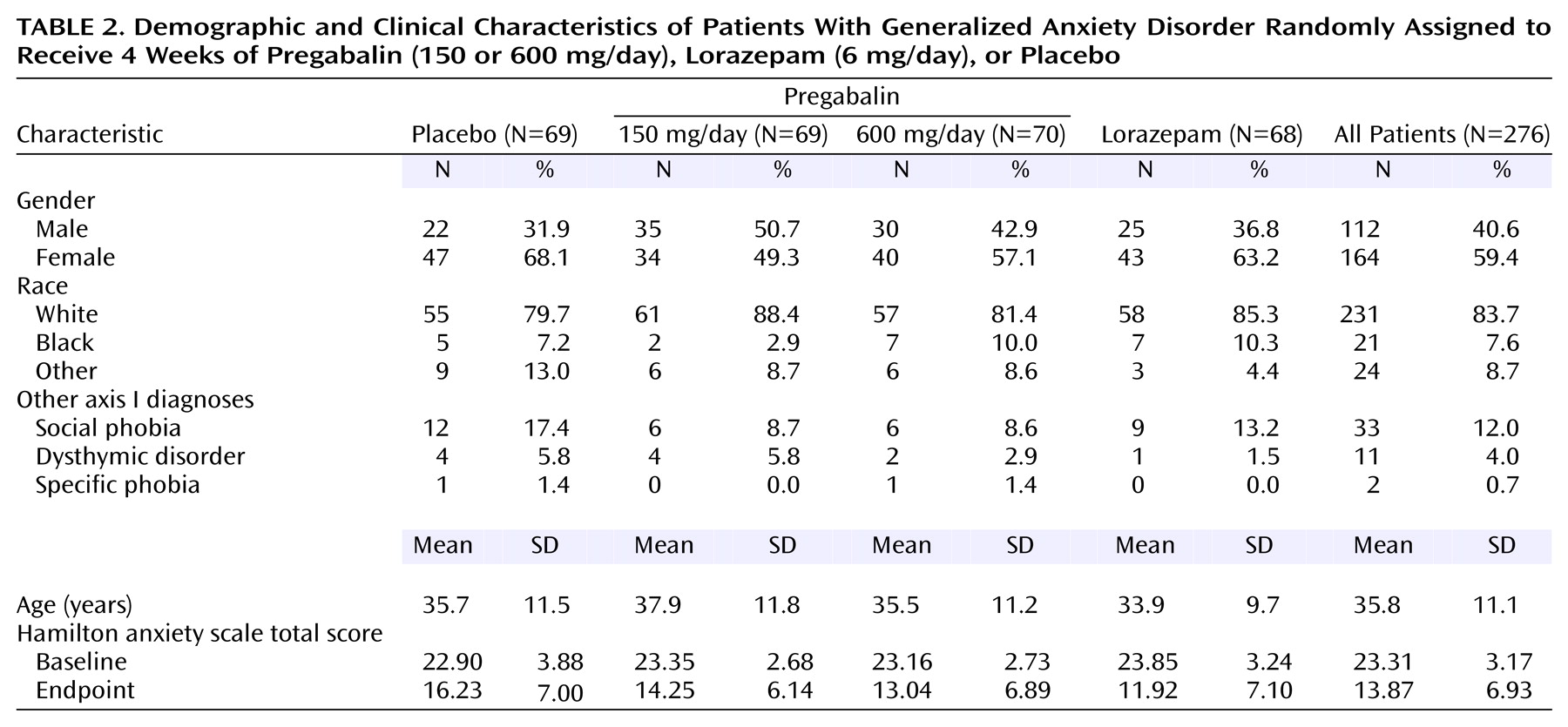
The four treatment groups were balanced with respect to patient demographic characteristics, with the exception of gender distribution (
Table 2). There were slightly more female subjects in the placebo and lorazepam groups. Age at onset of generalized anxiety disorder and duration of illness were similar across treatment groups. A low level of comorbidity was observed; social phobia and dysthymic disorder were the main comorbid diagnoses. The frequency of comorbid social phobia was slightly greater in the placebo group than in the other three treatment groups (
Table 2).
The use of concurrent psychotropic medication (mostly zolpidem) was infrequent (10 patients) and distributed evenly among treatment groups and therefore was not expected to affect the results.
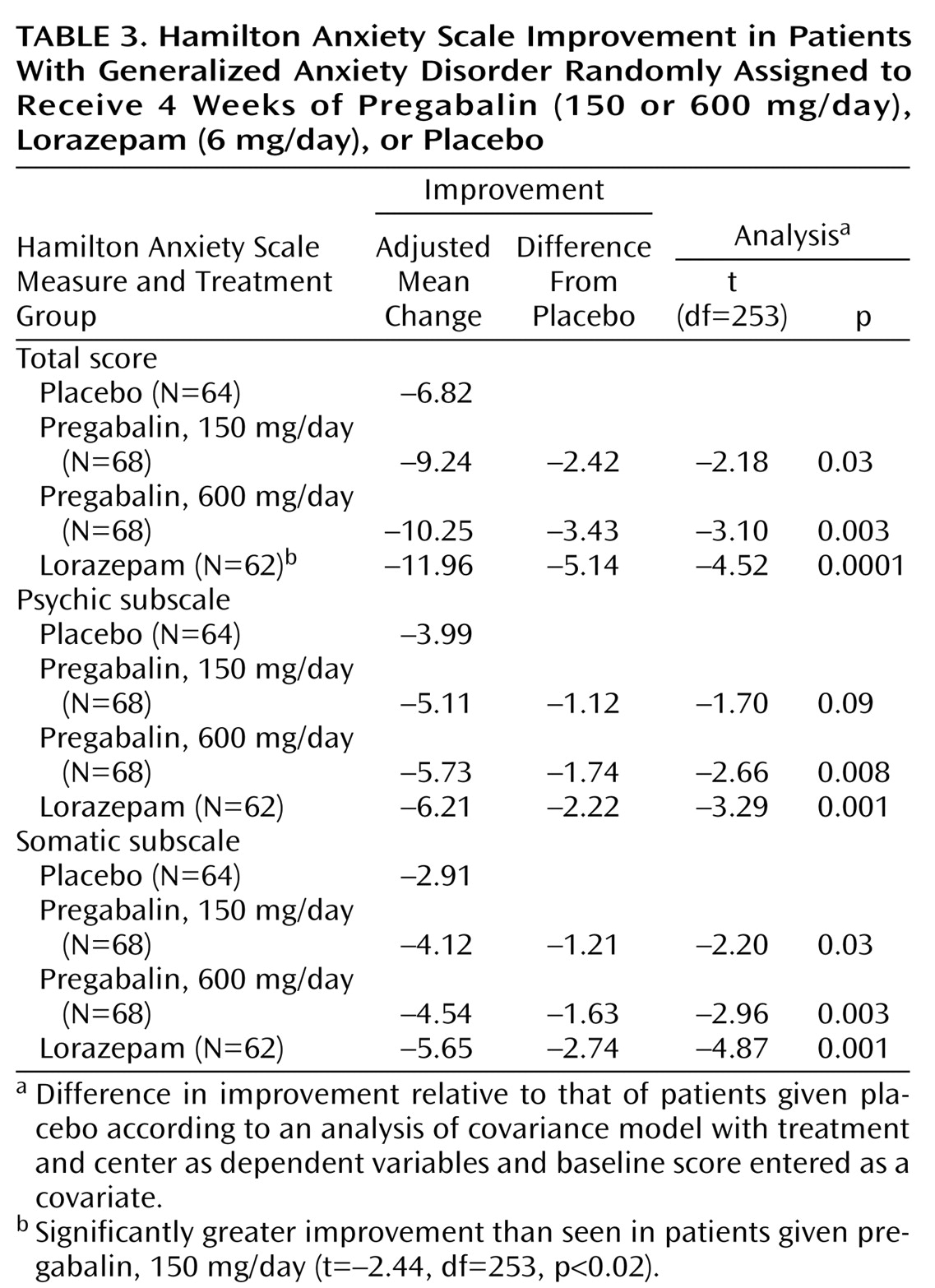
Of the 276 patients in the intent-to-treat population, 14 patients were missing postrandomization efficacy assessments (placebo group: N=5; pregabalin, 150 mg/day, group: N=1; pregabalin, 600 mg/day, group: N=2; lorazepam group: N=6). These patients were excluded from efficacy evaluations. Baseline severity of anxiety symptoms (as measured by the total Hamilton anxiety scale score) and of depressive symptoms (as measured by the total Hamilton depression scale score) did not differ between treatment groups. Hamilton anxiety scale total scores decreased in all treatment groups during the study. The baseline-to-endpoint decreases in Hamilton anxiety scale scores were significantly greater for patients given pregabalin, 150 mg/day, pregabalin, 600 mg/day, and lorazepam than for those given placebo (
Table 3). The difference in Hamilton anxiety scale change score between lorazepam and pregabalin, 150 mg/day, favored lorazepam. There was no significant difference in Hamilton anxiety scale change score for patients given 150 versus 600 mg/day of pregabalin (t=0.93, df=253, p<0.36) or for those given lorazepam versus pregabalin, 600 mg/day (t=–1.53, df=253, p<0.13). Efficacy results were not influenced by age or gender distribution.
In addition to the Hamilton anxiety scale total score, we examined the psychic and somatic subscales of the Hamilton anxiety scale. All three active treatments significantly reduced scores on the Hamilton anxiety scale somatic subscale compared with placebo (
Table 3). Pregabalin, 600 mg/day, and lorazepam significantly reduced scores on the psychic subscale compared with placebo, while treatment with pregabalin, 150 mg/day, resulted in a lower score that approached significance (
Table 3).
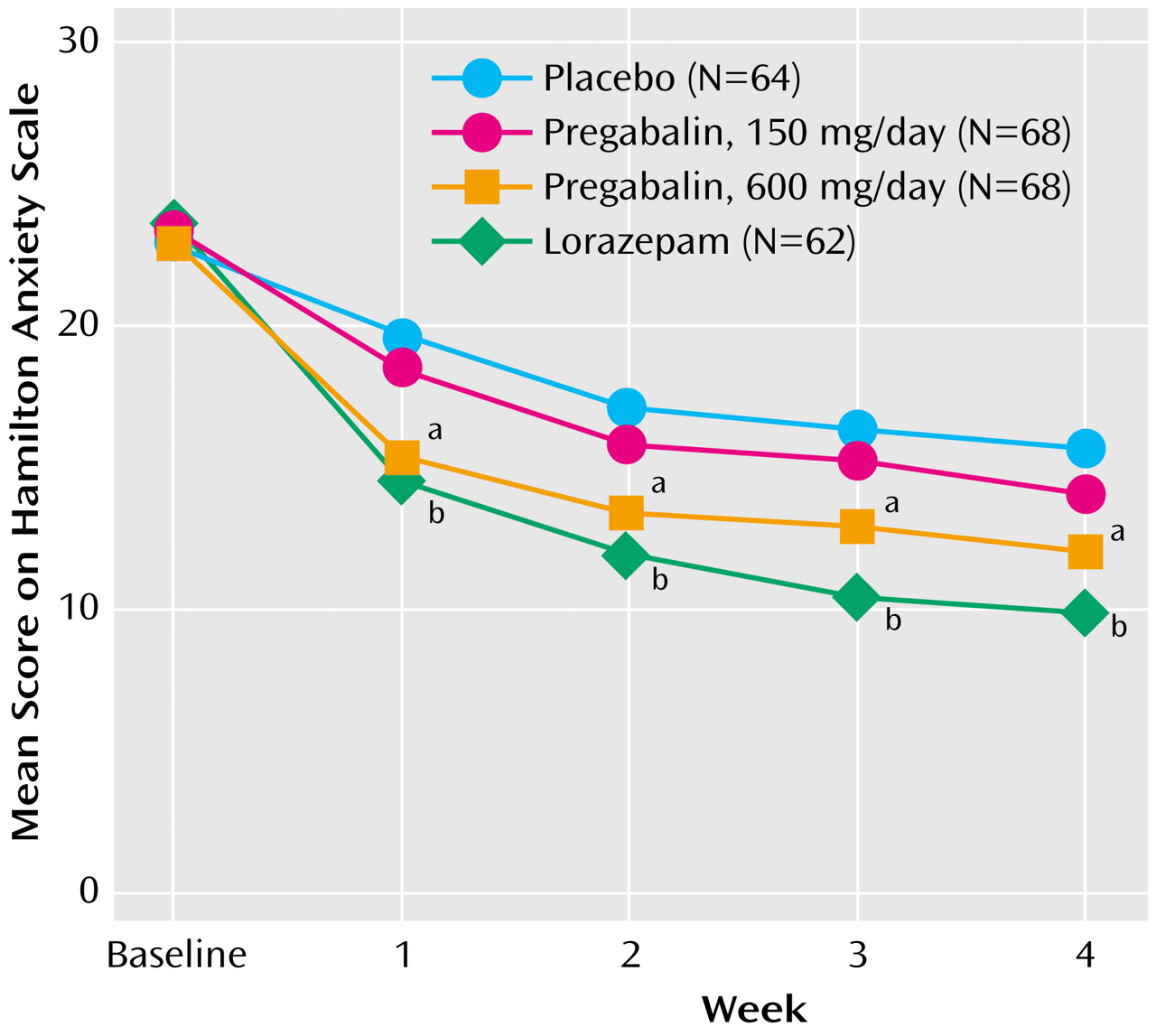
An analysis of observed cases by week showed that relative to placebo, both pregabalin, 600 mg/day, and lorazepam rapidly reduced the mean Hamilton anxiety scale total scores, even by the first week (
Figure 1).
Mean baseline Hamilton depression scale scores were fairly low for all groups. However, relative to placebo treatment, there was a significantly greater decrease from baseline to endpoint in the total Hamilton depression scale score for patients receiving pregabalin, 150 mg/day (t=–2.07, df=238, p<0.04); pregabalin, 600 mg/day (t=–3.30, df=238, p<0.002); and lorazepam (t=–2.79, df=238, p<0.006). Examination of the individual items of the Hamilton depression scale suggested that the effect was mainly due to changes in the anxiety and insomnia items.
In terms of Hamilton anxiety scale score, there were significantly more responders (≥50% decrease in score) among patients receiving pregabalin, 600 mg/day (46%, N=31 of 68) and lorazepam (61%, N=38 of 62) than among those given placebo (27%, N=17 of 64) (pregabalin, 600 mg/day, versus placebo: χ2=5.42, df=1, p<0.05; lorazepam versus placebo: χ2=15.11, df=1, p<0.05). Similarly, in terms of the CGI change rating, there were significantly more patients with ratings of “much improved” or “very much improved” among those receiving pregabalin, 600 mg/day (47%, N=32 of 68) and lorazepam (57%, N=35 of 62) than among those given placebo (28%, N=18 of 64) (pregabalin, 600 mg/day, versus placebo: χ2=5.75, df=1, p<0.05; lorazepam versus placebo: χ2=10.82, df=1, p<0.05). There were no significant differences in the numbers of responders for either definition of response between patients receiving pregabalin, 150 mg/day, and those given placebo.
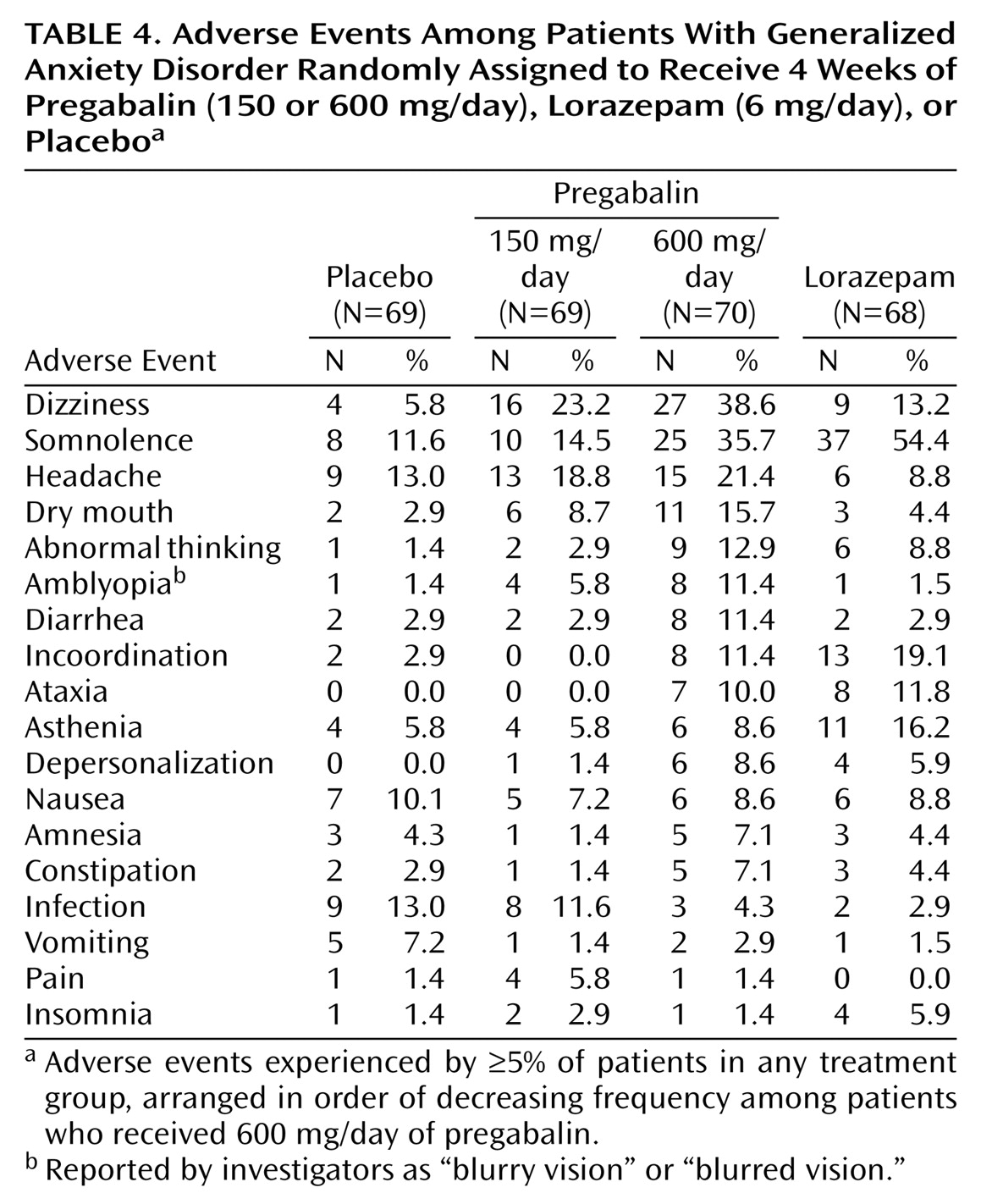
Adverse Events
Most patients in all treatment groups (78%) experienced an adverse event during the trial. Most patients reported adverse events that were moderate (42%) or mild (26%) in intensity. No pregabalin-treated patients experienced a serious adverse event. One patient receiving lorazepam experienced a serious adverse event (asthma attack) that was judged not related to study medication. A summary of adverse events by frequency is shown in
Table 4.
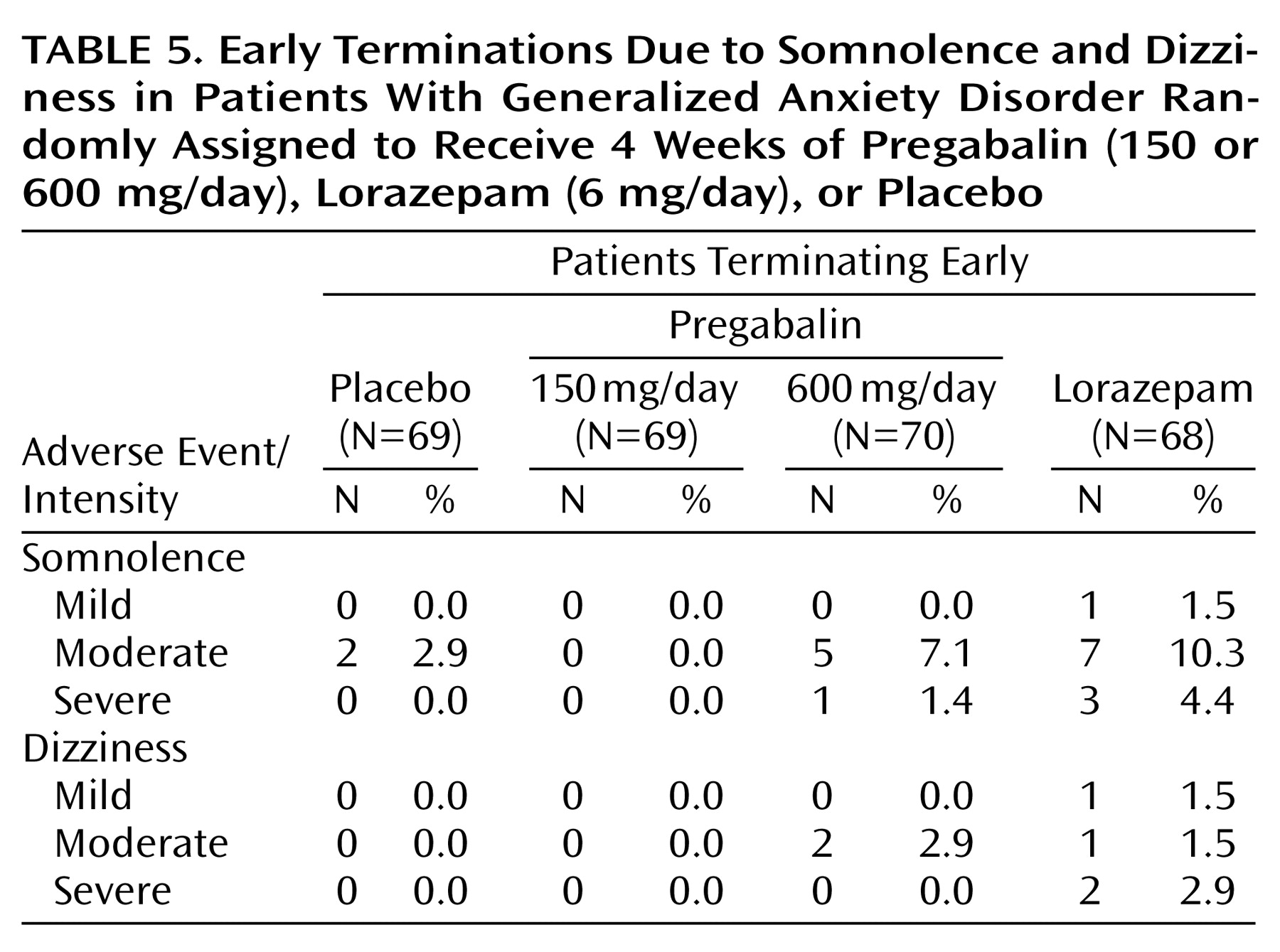
The most frequent events in the pregabalin groups were nervous system related. Dizziness was the most frequently occurring adverse event in both pregabalin groups. A total of 43 patients (30.9%) receiving pregabalin reported dizziness, which was usually mild (N=26) or moderate (N=15) in intensity. Somnolence was the most frequently occurring adverse event in the lorazepam group (N=37). Early terminations due to dizziness and somnolence were more frequent in the lorazepam group (
Table 5). This difference may be accounted for in part by the greater severity of the somnolence and dizziness among patients receiving lorazepam. There were no early terminations due to adverse events among patients receiving pregabalin, 150 mg/day.
Patients receiving pregabalin, 150 mg/day, experienced a mean weight gain of 1.3 kg (SD=1.7), while patients given 600 mg/day gained a mean 2.2 kg (SD=2.1), which was significantly more than the weight gain seen in those given placebo (mean=0.6 kg, SD=1.7) (t=4.58, df=227, p=0.0001). Patients receiving lorazepam had a mean weight loss of 0.2 kg (SD=1.8), which was significantly different than the weight change noted in the placebo group(t=–2.47, df=227, p=0.01). Three patients who received pregabalin, 600 mg/day, experienced weight gains of ≥7% from baseline to end of treatment. One patient receiving pregabalin, 150 mg/day, experienced a weight loss of ≥7% from baseline to end of treatment.
Withdrawal Effects
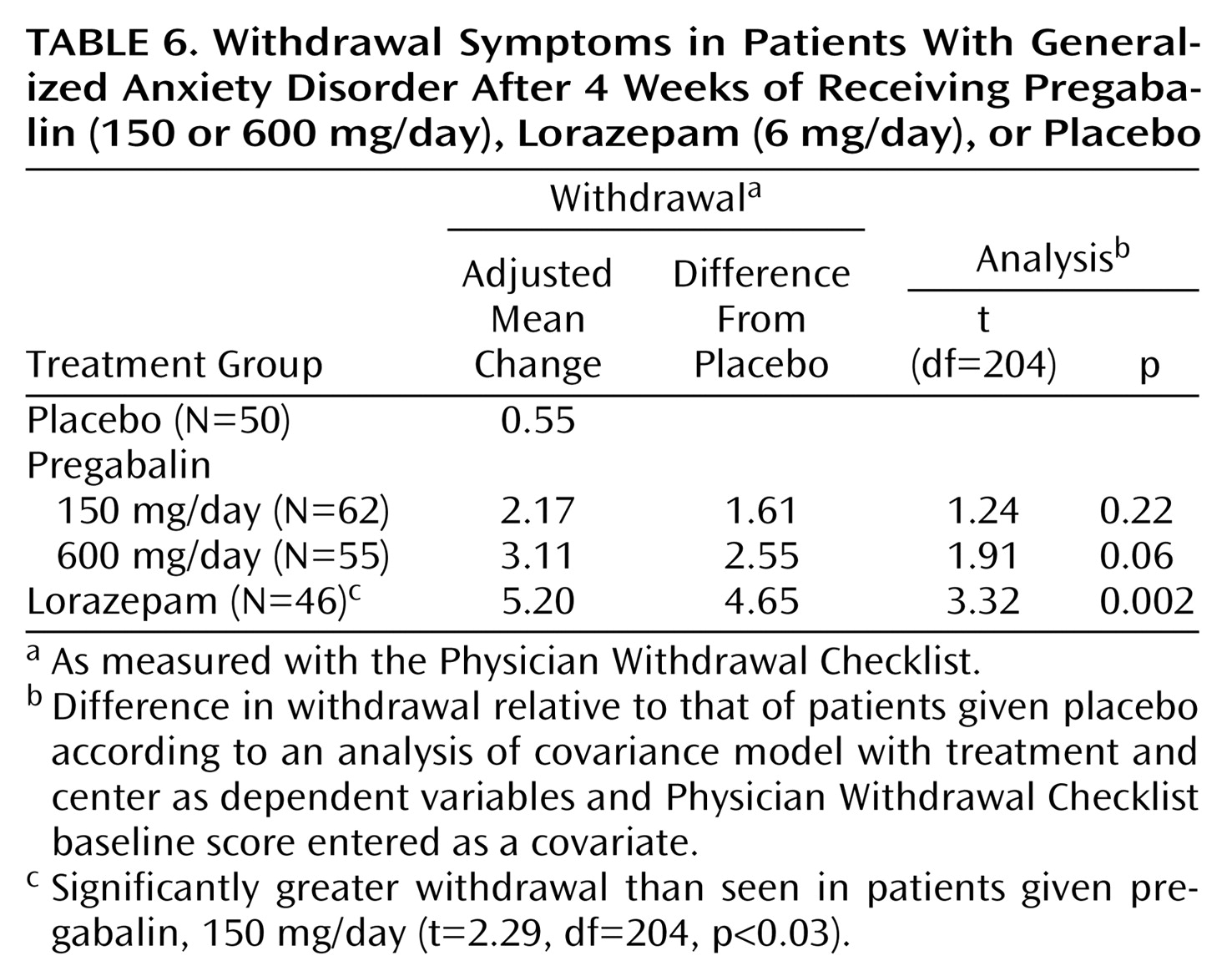
Symptoms of withdrawal from study medication were evaluated by the Physician Withdrawal Checklist change score. A positive Physician Withdrawal Checklist change score represents withdrawal effects or reemerging anxiety symptoms. The Physician Withdrawal Checklist change scores for both doses of pregabalin and lorazepam 6 mg/day were greater than that of placebo. However, only the lorazepam 6 mg/day Physician Withdrawal Checklist change score was significantly different from placebo (
Table 6).
Discussion
This study demonstrates that pregabalin is more effective than placebo in reducing the symptoms of anxiety as measured by the Hamilton anxiety scale among patients with generalized anxiety disorder. The antianxiety effect of pregabalin was detectable as early as 1 week after initiation of treatment. The anxiolytic effect of pregabalin, 600 mg/day, was comparable to that of lorazepam, 6 mg/day, in terms of the magnitude of change on the total Hamilton anxiety scale score and the speed of onset of anxiolytic effect. This is encouraging, since it suggests that the efficacy profile of pregabalin is comparable to that of lorazepam, a commonly used anxiolytic. Furthermore, discontinuation of pregabalin did not cause significant withdrawal effects.
All treatments were associated with some adverse events during the present trial. The most frequently occurring adverse events experienced by patients treated with pregabalin were somnolence and dizziness, and their frequency was dose-related. Since the upward titration of study drug followed a predetermined schedule, it is possible that a slower rate of titration might have lessened the frequency or severity of somnolence and dizziness. The onset of somnolence and dizziness was within the first few days of dosing, and these events were often transient in nature among those patients who continued in the study. Early terminations due to these two adverse events in the pregabalin groups were less frequent compared with the lorazepam group. This may indicate that even though patients receiving pregabalin, 600 mg/day, and lorazepam experienced qualitatively similar adverse events, lorazepam was less well tolerated. This is not unexpected given that a high dose of lorazepam was used in this study. However, the lorazepam dose selection was motivated by the need to compare the highest dose of pregabalin with the highest recommended dose of lorazepam. The present study, therefore, provides an initial calibration of the tolerability of pregabalin against a widely used anxiolytic.
Clinical experience suggests that with many anxiolytic drugs, rapid or sudden discontinuation of treatment may be associated with either a return or rebound of anxiety symptoms or, as in the case of the benzodiazepines, a characteristic abstinence syndrome. Analysis of data from the Physician Withdrawal Checklist in the present study indicated that among the three active anxiolytic treatments, only lorazepam, 6 mg/day, was associated with statistically significant withdrawal effects relative to placebo. These data and the similar rates of associated adverse events during the withdrawal phase across treatment groups indicate that no clear, prominent withdrawal syndrome was associated with pregabalin in this study. The likelihood of withdrawal effects emerging following drug discontinuation increases with increasing duration of treatment and more abrupt discontinuation. Therefore, the question of possible withdrawal effects associated with pregabalin will require further evaluation following longer treatment and more rapid treatment discontinuation.
This is the first report of the efficacy of pregabalin in generalized anxiety disorder and will require confirmation. The doses of pregabalin used in the trial were intended to define the boundaries of the likely risk-benefit profile. In future studies, doses between those studied in this trial may provide even better tolerability with equivalent efficacy to the 600 mg/day dose. Efficacy in this study was assessed after 4 weeks of treatment, which was sufficient to distinguish active drug from placebo. Longer-term studies will be required to assess the long-term safety and efficacy of pregabalin for the treatment of generalized anxiety disorder. In short-term treatment, most adverse events associated with pregabalin tended to resolve. It would be useful to know if the adverse events continue to dissipate with longer drug exposure, as is the case with many other psychotropic agents. Longer-term studies are currently underway to establish whether the anxiolytic effect of pregabalin is sustained over several months of continued treatment.
The present study included a population of patients whose predominant disorder at the time of presentation was generalized anxiety disorder. Most comorbid illnesses were excluded. In particular, although major depressive disorder was excluded, patients with mild depressive symptoms were permitted to enter. The observation that pregabalin significantly reduced Hamilton depression scale scores compared with placebo indicates that the relief of anxiety symptoms was not at the cost of worsening depression. Whether pregabalin is an effective anxiolytic in generalized anxiety disorder patients who have other comorbid psychiatric diagnoses is a question that must be addressed through additional studies.
The demonstration of anxiolytic efficacy of pregabalin in a population of patients with moderate to severe generalized anxiety disorder (based on Hamilton anxiety scale scores) is an important milestone. This compound represents a new class of anxiolytic agents. Although preliminary experiments suggested enhancement of GABA function by pregabalin
(22), subsequent data indicate that pregabalin is inactive at GABA
A and GABA
B receptors, it is not metabolized into GABA or a GABA antagonist, and it does not alter GABA uptake or degradation
(23,
24). Pregabalin reduces the release of several neurotransmitters, including glutamate, noradrenaline, and substance P
(25–
28), possibly through potent binding to the α
2δ subunit
(29) of the voltage gated calcium channel. Pregabalin binding at this site reduces calcium influx in nerve terminals and likely results in the analgesic, anxiolytic, and anticonvulsant activity exhibited by pregabalin.
Finally, pregabalin is also currently under study for treatment of seizure disorders and neuropathic pain. Preclinical and clinical data in these conditions suggest that pregabalin may have activity in several nervous system disorders other than anxiety. Therefore, further understanding of the pharmacology of pregabalin may provide new research leads in clinical neuroscience.
In conclusion, pregabalin may offer clinicians a safe and effective treatment option for patients with generalized anxiety disorder without potential for withdrawal effects.