The potential association between omega-3 fatty acids and psychiatric disorders has received considerable interest
(1–
5). The family of omega-3 fatty acids includes eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), but it is unclear at this time whether there are differences in their potential roles in treating depression or other psychiatric disorders. We report the results of a randomized, double-blind, placebo-controlled trial of the omega-3 fatty acid DHA in the treatment of participants experiencing a nonpsychotic major depressive episode.
Method
Adults (18 to 65 years of age) who met the DSM-IV criteria for major depressive disorder without psychotic features, established by clinical interview and the mood disorder module of the Structured Clinical Interview for DSM-IV, were considered for participation. The inclusion criteria were 1) a score of 12 or higher on the Montgomery-Åsberg Depression Rating Scale
(6), 2) a score of 17 or higher on the 28-item Hamilton Depression Rating Scale, 3) no psychotropic medication for at least 2 weeks, 4) dietary intake of no more than one serving of fish per week. The exclusion criteria included 1) significant comorbid psychiatric or medical illness and 2) treatment resistance, defined as a lifetime failure of two or more adequate antidepressant trials. The local institutional review board approved this study. All participants signed written informed consent statements after a full explanation of the study procedures, risks, and benefits.
A psychiatric and medical history was performed at the baseline visit. The Montgomery-Åsberg Depression Rating Scale was the a priori primary outcome measure, and response was defined as a 50% or greater reduction in score from baseline to study endpoint. The participants were randomly assigned to receive either 2 g/day of DHA or placebo for 6 weeks. Follow-up assessments were performed 2 and 6 weeks after the subjects began taking the study compound.
Two-sample t testing was used to examine differences in means between groups at baseline and for pretreatment and posttreatment comparisons of primary and secondary efficacy measures in the two groups. Fisher’s exact test was used to analyze differences in dichotomous variables between groups at baseline and to compare responder and remission status in the two groups.
Results
Of the 36 participants who enrolled, 35 had at least one follow-up assessment and were included in this intent-to-treat analysis. There were no significant differences between the two groups in age (DHA: mean=46.8 years, SD=11.6; placebo: mean=47.9 years, SD=11.2), gender (DHA: 77.8% women, N=14; placebo: 82.4% women, N=14), alcohol intake (DHA: mean=1.7 drinks per week, SD=3.8; placebo: mean=3.6 drinks per week, SD=5.8), or education level (DHA: mean=16.5 years, SD=2.4; placebo: mean=16.1 years, SD=3.3). However, the placebo group had a higher number of smokers (N=4) than the DHA group (N=0) (p<0.05, Fisher’s exact test) and a lower weight (placebo: mean=155.8 lb, SD=39.3; DHA: mean=189.7 lb, SD=55.7) (t=–2.07, df=33, p=0.05).
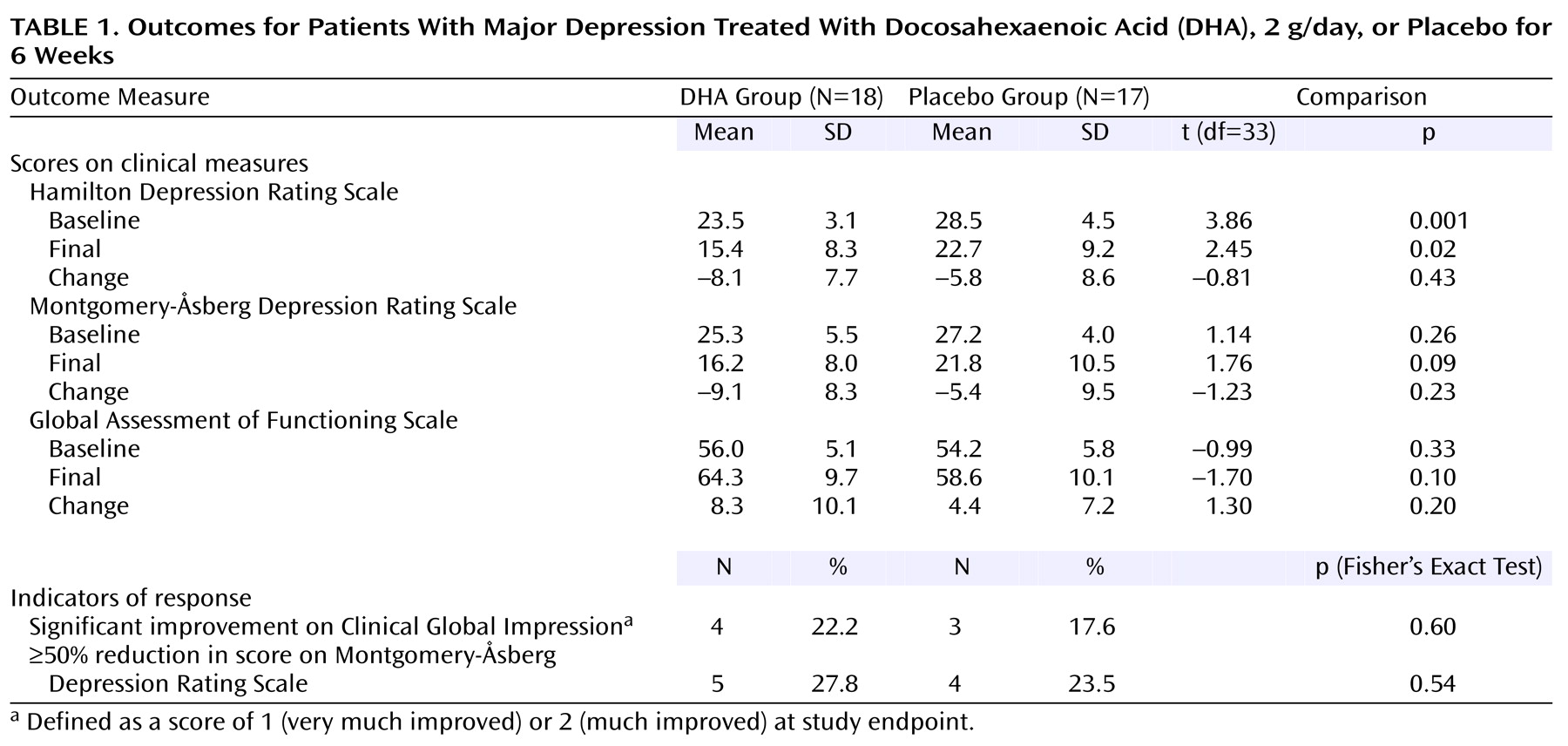
Results on the study outcome measures are shown in
Table 1. The rates of response according to the change in the score on the Montgomery-Åsberg Depression Rating Scale (primary outcome measure) were similar in the two groups, and the difference was not statistically significant. The difference remained nonsignificant after adjustment for the baseline Hamilton depression scale scores using analysis of covariance (F=0.63, df=1, 32, p=0.44).
The reported adverse events in the DHA group included a “fish” aftertaste (N=14), belching (N=3), lightheadedness or dizziness (N=3), loose stools (N=2), headache (N=2), and insomnia (N=1). The adverse events reported by participants in the placebo group included fatigue (N=3), insomnia (N=1), and loose stools (N=1). No participants were hospitalized or developed suicidal behavior, and none withdrew because of adverse events.
Compliance was confirmed by a twofold increase (t=8.40, df=19, p<0.0001) in red blood cell DHA levels among the patients receiving DHA treatment, while there was no change in DHA levels in the placebo group. The mean red blood cell DHA levels in the DHA-treated group, reported as percent by weight of the total fatty acids present in the sample, were 4.15% (SD=0.30) at baseline and 8.34% (SD=1.62) at endpoint, as compared to 3.78% (SD=1.05) at baseline and 3.86% (SD=1.14) at endpoint in the placebo-treated group.
Discussion
This controlled trial did not show a significant difference between DHA monotherapy and placebo in the treatment of adult outpatients with nonpsychotic major depression. To our knowledge, this is the first placebo-controlled monotherapy study of omega-3 fatty acids in the treatment of unipolar major depressive episodes.
Several methodological issues merit consideration. The randomization produced some baseline differences between the two groups. It is conceivable that these differences contributed to the results of the study in an as yet not understood manner. It is possible that 2 g/day is not optimal for the treatment of major depression or that the treatment period of 6 weeks was inadequate. We chose this dose and duration because of their feasibility when translated to routine clinical practice. Also of note is that, unlike patients in other studies
(7), the patients in this study did not appear to have lower DHA levels than normal subjects from a similar geographical region
(8). A lack of DHA deficiency, despite selection for low fish intake in this group, may also partially explain the lack of therapeutic benefit in the DHA treatment group. Finally, this study involved the use of only DHA and not EPA or other omega-3 fatty acids. It is unclear at this time whether one particular omega-3 fatty acid is potentially more effective than others in the treatment of major depression or whether the combination of EPA and DHA is potentially more effective in the treatment of major depression than either DHA or EPA alone.
This study was originally designed to detect a difference between groups in the change in Montgomery-Åsberg Depression Rating Scale score from baseline to endpoint. The expected mean difference between groups was 5 points (SD=5) with a power of 0.80. The difference that we actually detected was 3.7 points (pooled SD=8.9) with a power of 0.22. The confidence interval for the mean difference between groups was –2.46 to 9.86, so the actual difference in change in scores between groups could be as large as 9.86 points.
Despite the lack of therapeutic response in this study, it is important to note that the rationale for the use of omega-3 fatty acids (both DHA and EPA) in the treatment of bipolar disorder is different from that in unipolar depression and is not affected by these results. Clearly, more studies are needed to establish the potential role for omega-3 fatty acids in the treatment of affective disorders.