Management of Bipolar Disorder During Pregnancy and the Postpartum Period
Abstract
Risks Associated With Mood-Stabilizing Agents During Pregnancy
Lithium
Organ dysgenesis
Intrauterine growth effects
Neurobehavioral teratogenicity
Neonatal toxicity
Use during pregnancy
Anticonvulsants
Valproate
Organ dysgenesis
Intrauterine growth effects
Neurobehavioral teratogenicity
Neonatal toxicity
Use during pregnancy
Carbamazepine
Organ dysgenesis
Intrauterine growth effects
Neurobehavioral teratogenicity
Neonatal toxicity
Use during pregnancy
Lamotrigine
Organ dysgenesis
Intrauterine growth effects
Neurobehavioral teratogenicity
Neonatal toxicity
Use during pregnancy
Other Anticonvulsants
First-Generation Antipsychotic Agents
Organ dysgenesis
Neonatal toxicity
Behavioral teratogenicity
Use during pregnancy
Second-Generation Antipsychotic Agents
Organ dysgenesis
Neonatal toxicity
Use in pregnancy
Calcium Channel Blockers
Benzodiazepines and Other Sedative Hypnotic Agents
Organ dysgenesis
Use during pregnancy
Intrauterine growth retardation
Neonatal toxicity
Behavioral teratogenicity
ECT
Organ dysgenesis
Use in pregnancy
Intrauterine growth effects
Neonatal toxicity
Neurobehavioral teratogenicity
Psychosocial Interventions
Treatment Planning for the Pregnant Patient With Bipolar Disorder
Preconception
Early Conception
Second and Third Trimester
Unplanned Pregnancy
Treatment During the Puerperium and While Breast-Feeding
Lithium
Valproate
Carbamazepine
Lamotrigine
First-Generation Antipsychotic Agents
Benzodiazepines
Conclusions
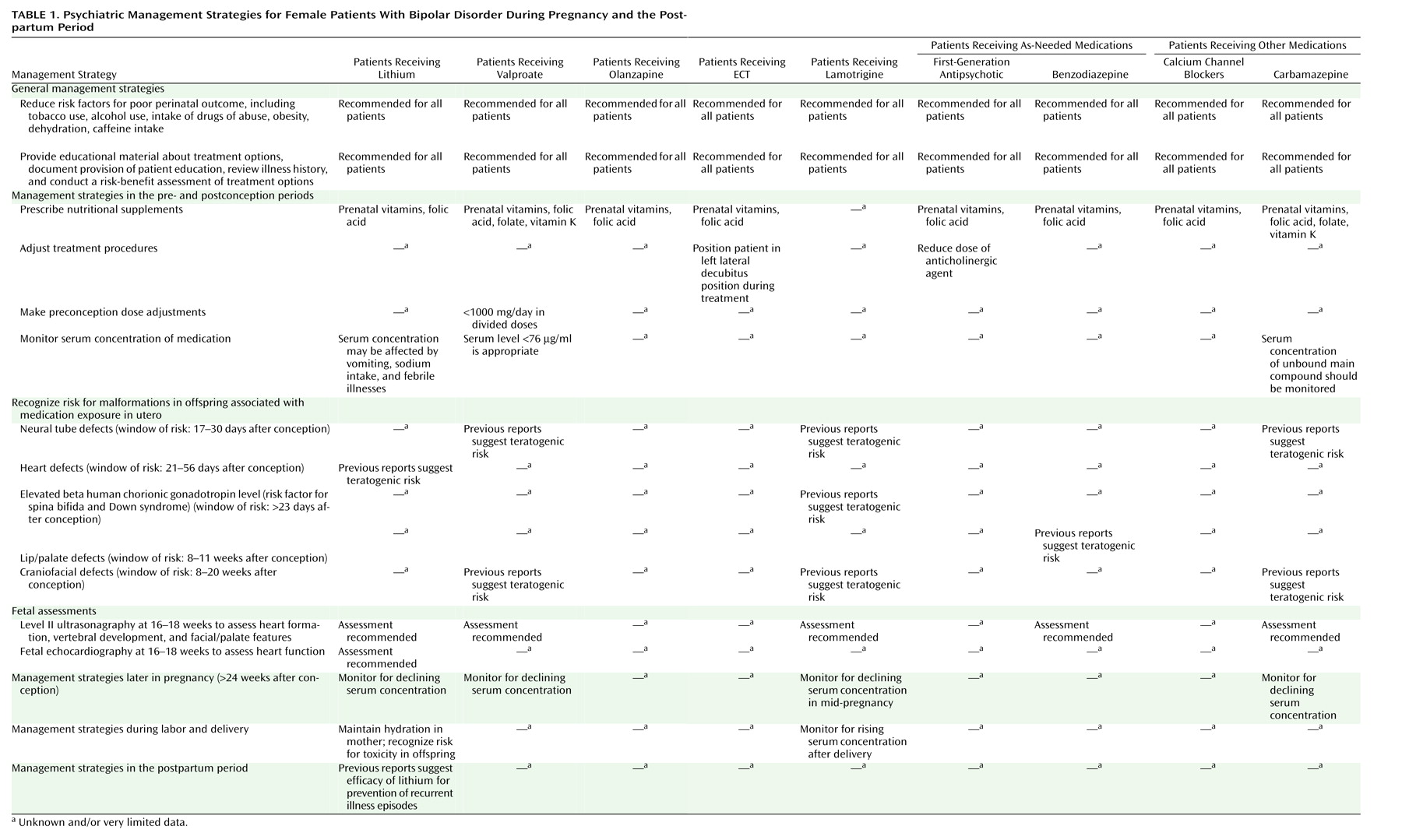
Footnote
References
Information & Authors
Information
Published In
History
Authors
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBGet Access
Login options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

