Attention deficit hyperactivity disorder (ADHD) is the most commonly diagnosed behavioral disorder of childhood
(1). Methylphenidate and amphetamine are the most frequently used treatments for ADHD
(1). Despite methylphenidate’s widespread use, its mechanisms of action are poorly understood. Methylphenidate blocks the dopamine transporters, and this indirect dopamine agonist effect may be critical for its therapeutic effects
(2). Specifically, stimulant-induced dopamine increases are believed to decrease background firing rates and increase signal-to-noise ratio of striatal cells
(3), which we postulate as a mechanism for improving attention by enhancing task-related neuronal cell firing. Since dopamine also modulates salience and motivation
(4), we postulate that small and brief task-induced dopamine increases would be amplified by methylphenidate, which would increase the salience of the task and thus improve performance
(5).
Using positron emission tomography (PET), we have shown that a relatively large therapeutic dose of oral methylphenidate (60 mg) significantly blocked dopamine transporters in all subjects but did not increase extracellular dopamine in all of them
(6). We reasoned that methylphenidate-induced increases in dopamine were due not only to dopamine transporter blockade but also to the rate of dopamine release, which is mostly a function of dopamine cell firing
(7). Since dopamine cells fire in response to salient stimuli, we postulated that methylphenidate-induced dopamine transporter blockade (the main mechanism for removal of extracellular dopamine
[8]) would amplify dopamine-induced increases to salient stimuli and thus be context dependent. In the present study we tested whether methylphenidate would enhance increases in extracellular dopamine in response to an academic task (solving mathematical problems with monetary reinforcement), relative to a neutral task (passively viewing scenery cards with no remuneration). We used a mathematical task because this type of task is frequently used as a measure to assess treatment efficacy in ADHD. Although in a classroom setting performance is not monetarily remunerated, it is often reinforced by grading
(9). We chose as the neutral task passive viewing of scenery pictures rather than a mathematical task with no remuneration because we wanted to optimize the contrast between a task that might maximize dopamine increases (mathematical task) and a task that would minimize dopamine increases (viewing neutral pictures).
We used PET imaging with [
11C]raclopride, a dopamine D
2 receptor ligand sensitive to competition with endogenous dopamine
(10), to measure the relative changes in extracellular dopamine induced by methylphenidate when administered before the mathematical task and before the neutral task. Because [
11C]raclopride binding is highly reproducible
(11), differences in binding between placebo and intervention predominantly reflect intervention-induced changes in extracellular dopamine
(12). Our a priori hypothesis was that methylphenidate, by blocking dopamine transporters, would amplify the dopamine increases induced by the task, thus enhancing its saliency.
Method
Subjects
Sixteen healthy subjects (14 men and two women; mean age=35 years, SD=8) selected from a pool of subjects who responded to an advertisement were studied. Subjects were initially screened by phone and then evaluated at Brookhaven National Laboratory by a physician (G.-J.W.) to ensure that there was 1) no current or past psychiatric or neurological disease, drug abuse, or significant medical illness; 2) no medications being taken (including over-the-counter medications); and 3) no pregnancy. Physical examination and laboratory test results were also obtained. Magnetic resonance imaging scans were not performed, so we could not corroborate a lack of structural brain abnormalities. On average, out of every five subjects initially screened one was recruited, and all of the 16 subjects recruited completed the study. Prescan urine tests ensured the absence of any psychoactive drug, including sedative drugs, and the absence of pregnancy in the female subjects. Subjects were monetarily compensated for their participation in the study. Written informed consent was obtained from all subjects after complete description of the study and following the guidelines set by the Institutional Review Board at Brookhaven National Laboratory.
Scans
PET scans were run on a whole-body, high-resolution positron emission tomograph (Siemens/CTI ECAT HR+, with 4.6×4.6×4.2 mm National Electrical Manufacturers Association resolution at center of field of view and 63 slices) in three-dimensional dynamic acquisition mode using [
11C]raclopride as a dopamine D
2 receptor ligand
(13). Methods for positioning of subjects, catheterizations, transmission scans, and blood sampling and analysis have been published
(11). Briefly, emission scans were started immediately after injection of 4–8 mCi of [
11C]raclopride (specific activity: 0.5–1.5 Ci/μmol at the end of bombardment). A series of 20 emission scans were obtained from time of injection up to 54 minutes. Arterial sampling was used to measure total carbon-11 and unchanged [
11C]raclopride in plasma.
Subjects were scanned four times with [
11C]raclopride under four conditions defined by the drug/task combinations: 1) neutral task preceded by placebo administration (placebo/neutral task); 2) neutral task preceded by methylphenidate administration (methylphenidate/neutral task); 3) mathematical task preceded by placebo administration (placebo/math task); and 4) mathematical task preceded by methylphenidate administration (methylphenidate/math task). Subjects were scanned on 2 days and were blinded as to whether they received methylphenidate or placebo. On one day the first scanning condition was placebo/neutral task and the second condition 2 hours later was methylphenidate/math task. On the other day, the first scanning condition was placebo/math task and the second condition 2 hours later was methylphenidate/neutral task. The order of these two sessions was balanced across subjects. The placebo (calcium carbonate) or methylphenidate (20 mg) was given orally 60 minutes prior to the mathematical and the neutral tasks, which were started 15 minutes prior to the injection of [
11C]raclopride. Venous blood was drawn for quantification of plasma concentrations of methylphenidate prior to and 60, 90, and 120 minutes after oral methylphenidate using capillary gas chromatography/mass spectrometry
(14).
Stimulation
For the mathematical task, the subjects were asked to answer a series of mathematical problems that were preselected for each subject so that they would respond correctly to 80% of them. This also required that the task difficulty be adjusted depending on performance during the study. Problems were presented in a series of colored cards at a rate of one card per minute. Correct responses were remunerated by 25 cents to one dollar depending on the difficulty of the question, which were chosen so that subjects would earn approximately 50 dollars per session. For the neutral task, subjects were presented with cards that showed images of scenery and were not asked to provide responses nor were they remunerated. The mathematical and the neutral tasks began 15 minutes prior to radiotracer injection and continued for 45 minutes.
Behavioral Measures
Subjects were asked to provide subjective ratings (from 1 to 10 for which 1=low and 10=high) of the mathematical task for the following descriptors: interesting, exciting, motivating, boring, and tiresome. These ratings were obtained just prior to [11C]raclopride injection and 15 minutes after initiation of the mathematical task. Heart rate and blood pressure were monitored continuously. The behavioral measures were lost for one of the subjects.
Image Analysis
Regions of interest were outlined for the striatum and cerebellum as described previously
(11). Briefly, regions of interest were initially outlined on the individual’s summed baseline [
11C]raclopride image (images obtained between 15 to 54 minutes) and were then projected into the dynamic [
11C]raclopride images to generate time activity curves for the striatum and cerebellum. These time activity curves for tissue concentration along with the time activity curves for unchanged tracer in plasma were used to calculate [
11C]raclopride’s transfer constant from plasma to brain (K
1) and the distribution volume, which corresponds to the equilibrium measurement of the ratio of tissue concentration to plasma concentration, in the striatum and cerebellum using a graphical analysis technique for reversible systems
(15). The ratio of distribution volume in the striatum to that in the cerebellum corresponds to (B
max/K
d)+1 and is insensitive to changes in cerebral blood flow
(16). Differences between conditions were quantified as percent change in B
max/K
d with respect to the placebo/neutral task condition, which was used as the baseline condition. The effects of methylphenidate on the mathematical task were quantified as percent change in B
max/K
d with respect to the placebo/math task condition.
Data Analysis
Differences in K1, distribution volume, and Bmax/Kd between conditions were tested using repeated analysis of variance (ANOVA), and post hoc t tests were then used to determine in which conditions the effects differed from the baseline condition (placebo/neutral task). The effects of methylphenidate on the descriptor ratings of the mathematical task were tested using paired t tests between the placebo/math task condition and the methylphenidate/math task condition. Pearson product-moment correlations were used to assess the relationship between the changes in dopamine between the placebo/math task condition and the methylphenidate/math task condition and the changes in the descriptor ratings for the two conditions.
Results
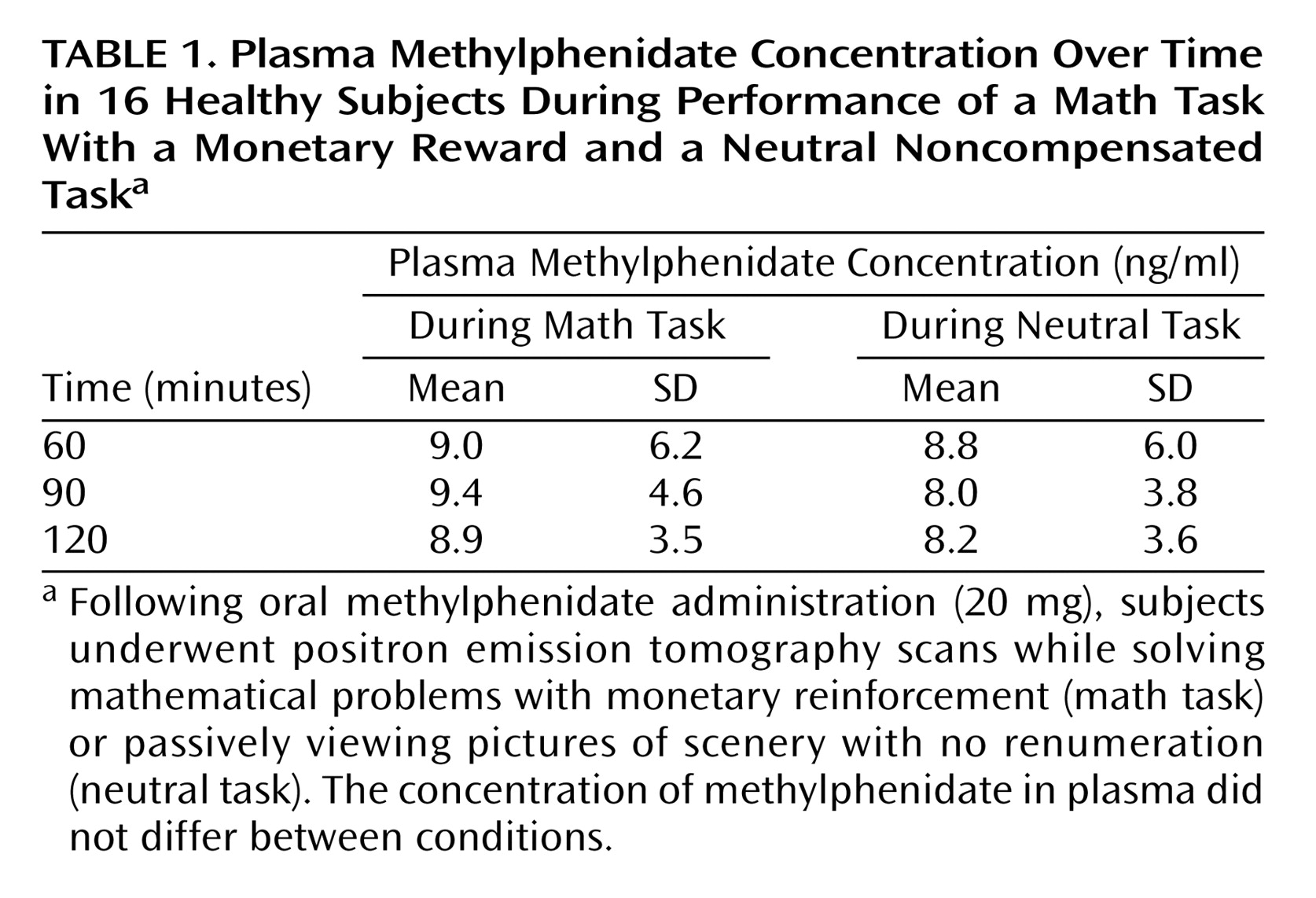
The plasma methylphenidate concentrations were the same in the methylphenidate/neutral task and the methylphenidate/math task conditions, and they were in the typical therapeutic range of about 8–9 ng/ml at the time when the PET [
11C]raclopride measures were obtained (
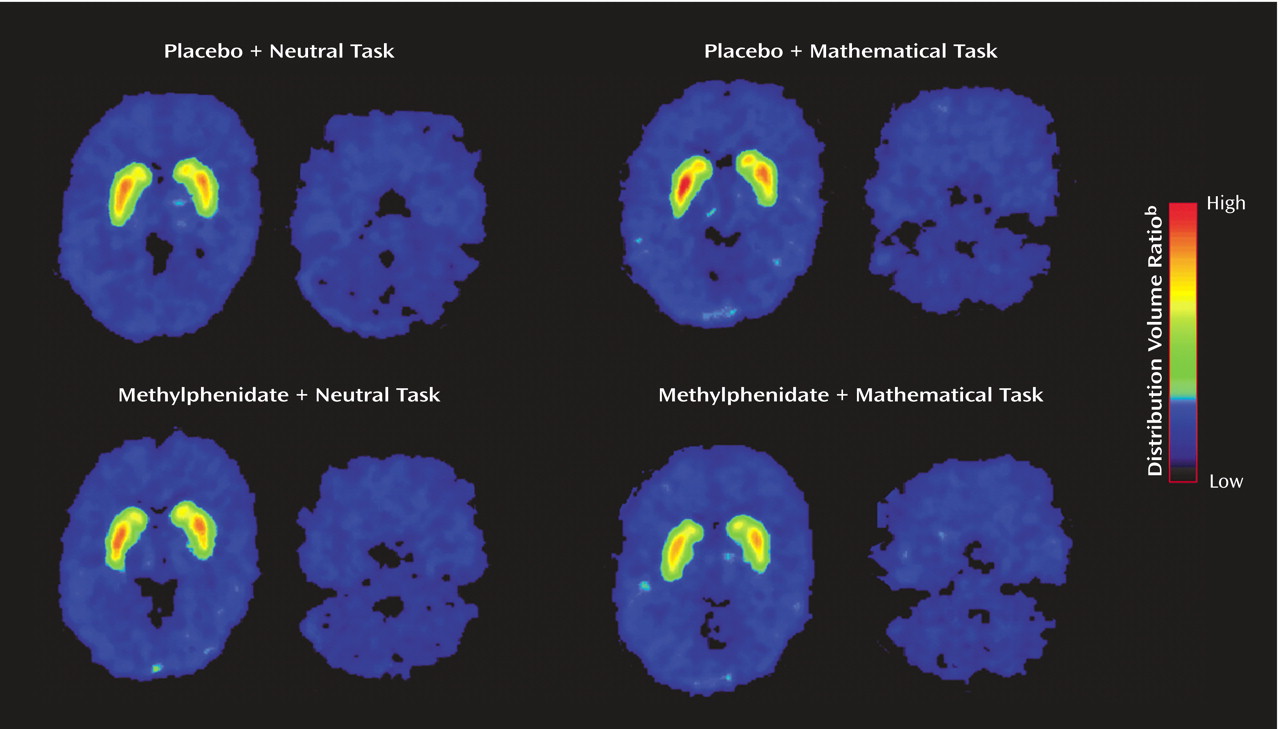
Table 1). The distribution volume images for one of the subjects are shown in
Figure 1 for the four testing conditions: placebo/neutral task (condition used as baseline), placebo/math task (assessing the effects of the mathematical task by itself), methylphenidate/neutral task (assessing the effects of methylphenidate by itself) and methylphenidate/math task (assessing the combined effects of methylphenidate and the mathematical task).
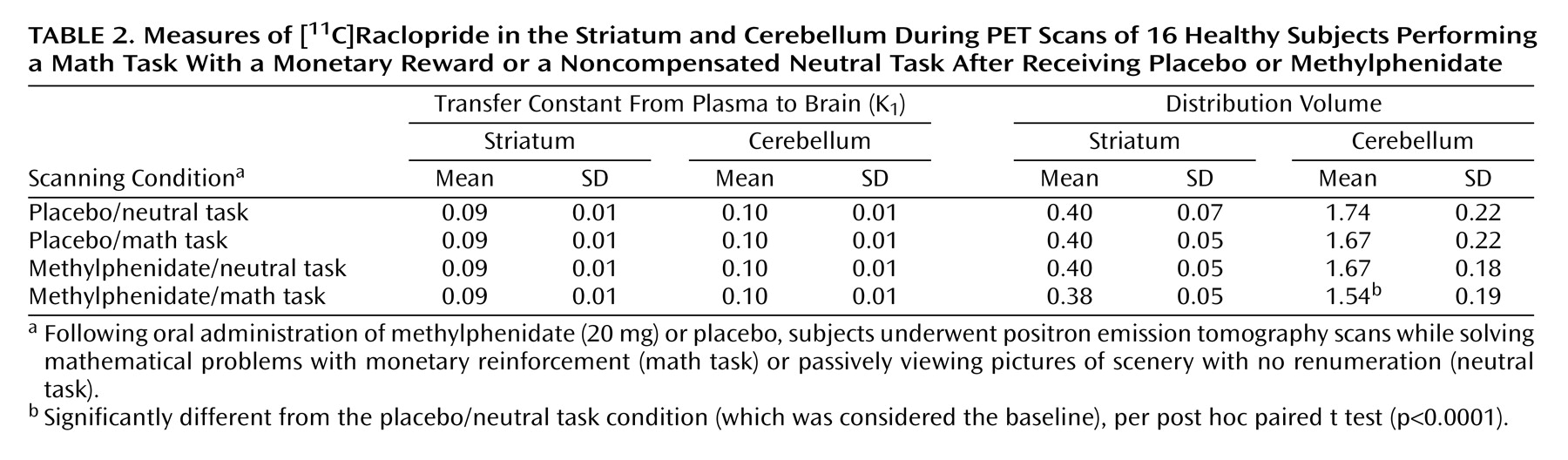
There were no differences across conditions in K
1 in the striatum or cerebellum, indicating that there were no significant condition-related differences in radiotracer delivery (
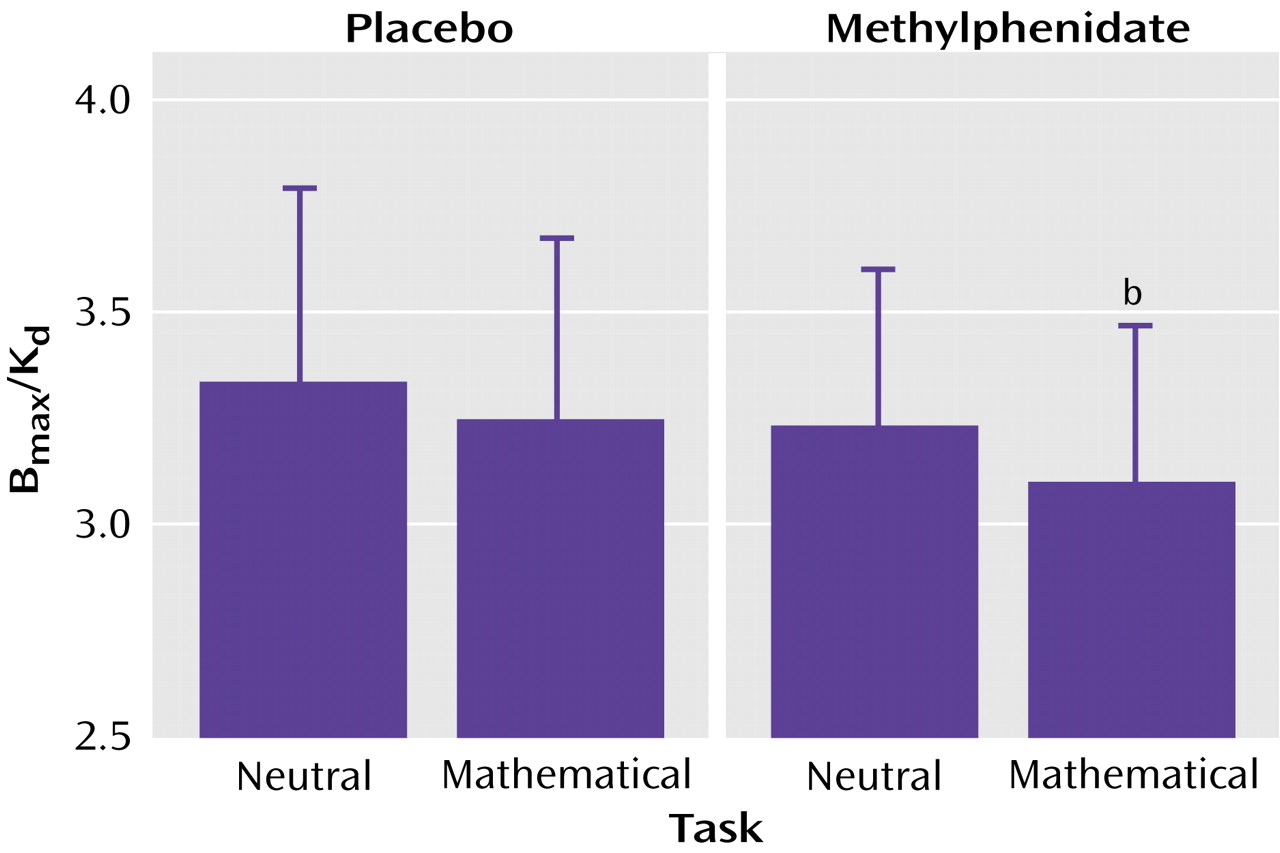
Table 2). There were no differences in the distribution volume in cerebellum. The repeated-measures ANOVA showed significant differences across the conditions for distribution volume in the striatum (F=8.2, df=3, 63, p<0.001). Post hoc t tests showed that when compared with the baseline condition, distribution volume measures in the striatum were significantly lower for the methylphenidate/math task condition (p<0.0001) but did not differ for the placebo/math task and the methylphenidate/neutral task conditions (
Table 2). Similarly, the repeated-measures ANOVA showed significant differences across the conditions for the striatal B
max/K
d measures (F=2.75, df=3, 63, p<0.05). Post hoc t tests showed that when compared with the baseline condition they were significantly lower for the methylphenidate/math task condition but did not differ for the placebo/math task and the methylphenidate/neutral task conditions (
Figure 2). This indicates that the only condition for which the dopamine changes were large enough to be detected by the [
11C]raclopride method was when methylphenidate was administered with the mathematical task.
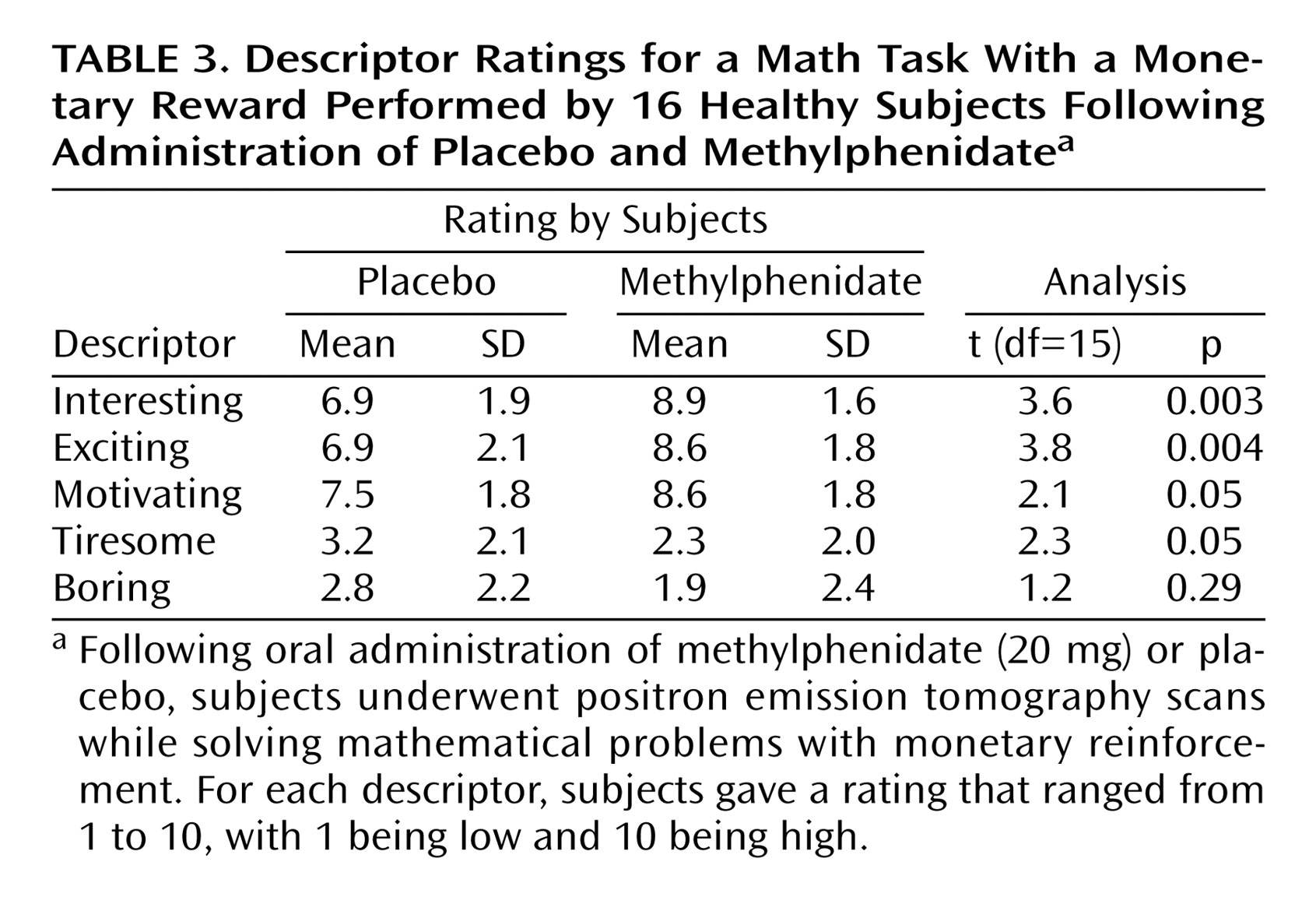
Analysis of the behavioral measures showed a significant effect of methylphenidate in the perception of the mathematical task by the subjects. Methylphenidate significantly increased the ratings of the task as being interesting, exciting, motivating, and less tiresome (
Table 3).
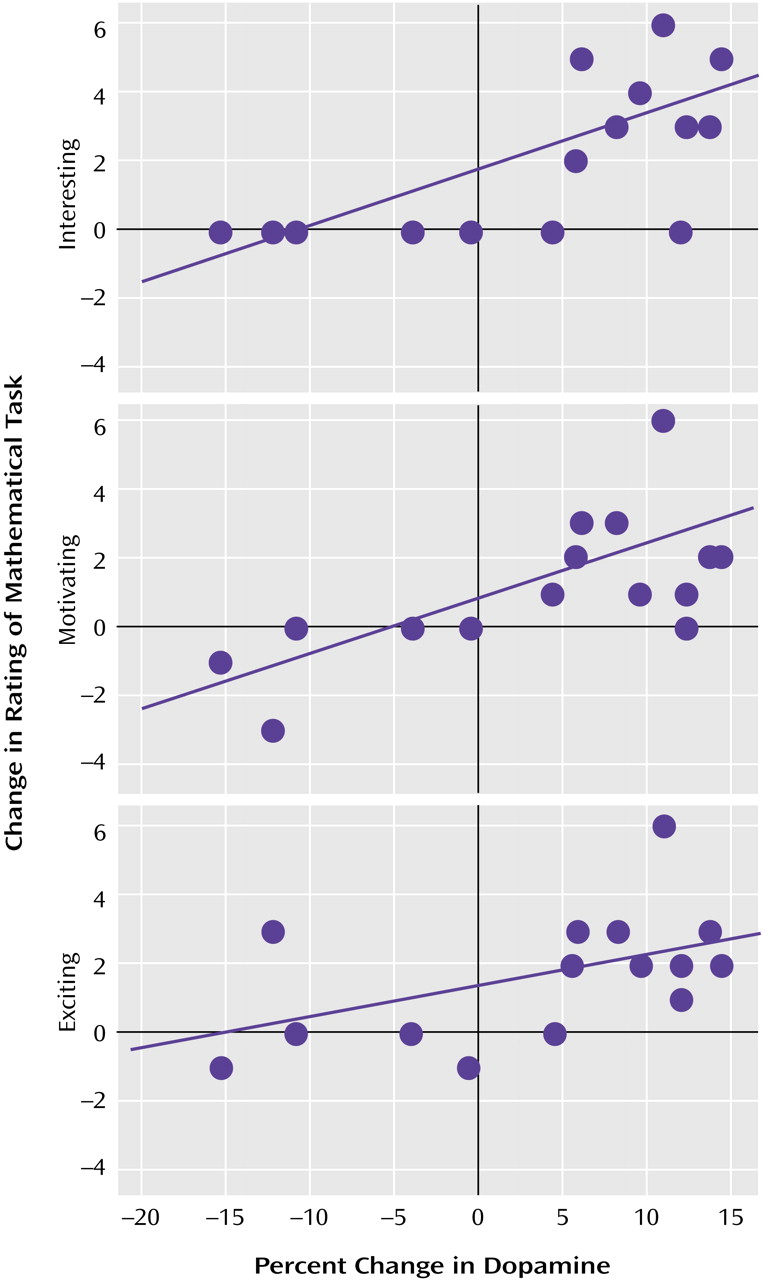
The correlation analyses showed a significant association between the changes in extracellular dopamine between the methylphenidate/math task and the placebo/math task conditions and the changes in the rating of interesting (r=0.67, df=14, p=0.006), exciting (r=0.52, df=14, p<0.05), and motivating (r=0.67, df=14, p=0.006) (
Figure 3).
The changes in Bmax/Kd during the methylphenidate/math task condition were not correlated with the plasma methylphenidate concentration (60 minutes: r=26, df=14, p=0.34; 90 minutes: r=0.33, df=14, p=0.21), indicating that differences in methylphenidate metabolism were not responsible for the variability in the dopamine increases. Correlations between the dopamine changes during the methylphenidate/math task conditions and age revealed a significant negative correlation: the younger the subjects, the larger the increases in dopamine (r=–0.68, df=15, p=0.004).
Discussion
Here we have shown that methylphenidate-induced increases in dopamine are context dependent. Methylphenidate increased extracellular dopamine in the striatum when administered before subjects were required to perform a remunerated mathematical task but not when administered before subjects passively viewed scenery cards. Since dopamine cell activity is sensitive to environmental stimulation (salient and novel stimuli activate dopamine cells
[17]), this suggests a mechanism to explain why methylphenidate-induced increase in dopamine depends on the conditions of its administration.
The context dependency of stimulant-induced dopamine increases has been demonstrated in laboratory animals for cocaine, which like methylphenidate increases dopamine by blocking the dopamine transporters
(18). For example, cocaine-induced increases in dopamine in the nucleus accumbens were larger when animals were given cocaine in an environment where they had previously received cocaine than when they received it in a novel environment
(19) or when animals self-administered cocaine than when cocaine administration was involuntary
(20). Also methylphenidate-induced increases in dopamine in the prefrontal cortex were significantly greater when rats were restrained at the time of administration than when they were not
(21).
In clinical studies, the context dependency of stimulant medication was one of the first effects noted in early studies of “hyperactivity”
(22) and has been replicated in subsequent studies in children with ADHD
(23). Recently, context dependency was documented in a laboratory school setting in which children with ADHD treated with methylphenidate showed larger reductions in placebo-adjusted activity level in the classroom than in the playground setting
(24). Similarly, in a previous PET study we showed that methylphenidate-induced increases in extracellular dopamine were larger when it was administered with a salient stimulus (visual display of food in food-deprived subjects) than with a neutral stimulus (recall of family genealogy)
(25).
In this study, the pairing of 20 mg of methylphenidate with the neutral task did not increase extracellular dopamine. This differs from a study in which we showed dopamine increases with 60 mg of methylphenidate when given with a neutral stimulus and is likely to reflect the much larger dose used for that study. However, the current findings also differ from a previous study in which we showed dopamine increases with 20 mg of methylphenidate when given with a neutral stimulus in food-deprived subjects
(25). This discrepancy is likely to reflect food deprivation, which has been shown to affect extracellular dopamine
(26). Further studies are required to assess if methylphenidate’s effects in the human brain are sensitive to acute food deprivation in ways other than that of the effects of food on drug absorption.
Methylphenidate significantly increased the descriptor ratings of interesting, exciting, and motivating for the mathematical task and showed a tendency to decrease the rating for the task descriptor tiresome. The increases in these descriptors were associated with methylphenidate-induced dopamine increases when given with the mathematical task. This suggests that the amplification of small dopamine increases due to the task by methylphenidate may be the mechanism underlying this drug’s ability to make tasks more salient to the subject. Although most of methylphenidate’s therapeutic effects have emphasized its effects on attention, here we document an effect of methylphenidate on motivation as reflected by the subjective report of how the subject experiences the task in terms of descriptors of interesting, exciting, and motivating, which we consider to be characteristics of salience in this setting. We postulate that methylphenidate’s ability to increase the saliency of the task will increase the motivation it elicits and that this will result in improved performance. Indeed, others have pointed to the potential relevance that methylphenidate’s effects on saliency may have on its therapeutic effects in ADHD
(27). Also, methylphenidate when used for other neuropsychiatric conditions has been shown to be effective in the treatment of apathy, which is an extreme state of lack of motivation
(28).
The levels of extracellular dopamine in the striatum are set by two processes: tonic dopamine cell firing (maintains baseline steady-state levels and sets the overall responsiveness of the dopamine system) and phasic dopamine cell firing (leads to fast dopamine changes that highlight the saliency of stimuli)
(29). It had been previously hypothesized that therapeutic effects of methylphenidate are due to increased tonic dopamine levels, which stimulate dopamine autoreceptors and attenuate phasic dopamine increases
(30). However, we now postulate that the long-lasting blockade of dopamine transporters by methylphenidate actually amplifies stimuli-induced dopamine increases (their magnitude and duration). Both the current results and those from a prior PET study in which we showed that methylphenidate amplified dopamine increases induced by display of food in food-deprived subjects
(25) support the notion of an amplification of dopamine increases by oral methylphenidate rather than attenuation. Thus we postulate that methylphenidate will operate to amplify the saliency value of stimuli to which the subject may be exposed during everyday routines and which by themselves may have been insufficient to elicit dopamine responses strong enough and lasting long enough to signal saliency and drive and maintain interest and attention for the period required to complete the relevant task.
In this study the mathematical task, even with monetary remuneration, was insufficient by itself to elicit a measurable response. This most likely reflects the limited sensitivity of the methodology. Although some studies have shown decreases in [
11C]raclopride binding in striatum with behavioral interventions
(31,
32), others have failed to show an effect after exercise
(33) or after food stimulation
(25). These differences most likely reflect the magnitude and duration of the dopamine changes elicited by the various behavioral interventions.
In this study methylphenidate, when given with the neutral task, was insufficient to elicit a measurable response. This most likely reflects in addition to the limited sensitivity of the methodology the relatively small dose used (20 mg for a group whose mean weight was 70 kg) and the lack of saliency of the neutral task. Note that we have reported increases with dopamine after 60 mg of methylphenidate when given in a neutral condition
(5) but have also reported increases with 20 mg when given with salient stimuli other than money
(25). These results offer a new hypothesis of why combining pharmacological and psychosocial interventions is more effective than medication alone. The traditional explanation is that a given dose of medication produces a specific effect by blocking dopamine transporters and increasing dopamine, which operates to focus attention and that this in turn enhances the impact of behavioral techniques (such as tokens earned for attending to an academic task in the classroom). However, if token reinforcers in behavioral interventions operate like money in the present study, then the behavioral intervention may operate to magnify the stimulant-induced increase in dopamine for a given dose of medication. This may explain why in the Multimodal Treatment Study of Children With ADHD
(34), the combination of behavior modification with medication produced the same or slightly superior reduction of symptoms at a lower dose of methylphenidate
(35).
Methylphenidate-induced increases in dopamine when co-administered with the mathematical task declined as a function of age. An age-related blunting in stimulant-induced dopamine increases has also been observed after intravenous
(36) and oral methylphenidate
(5) and after intravenous amphetamine in healthy subjects
(37). This could reflect a decrease in dopamine release with aging. Indeed, microdialysis studies have shown that stimulant-induced increases in extracellular dopamine are significantly lower in older than in younger animals
(38,
39).
The following are study limitations. First, although changes in [
11C]raclopride binding are linearly related to extracellular dopamine, the precise relationship with synaptic dopamine is not understood
(10). Second, to avoid the need for four arterial cannulations, we conducted the studies over 2 rather than 4 days. This required us to always give methylphenidate during the second study of the day. However, the order effect cannot explain the differences observed with methylphenidate when given with the neutral versus the mathematical task, since for both conditions methylphenidate was given at the same time of day. Third, the experimental design did not allow us to evaluate the effects of methylphenidate on performance, since the difficulty of the problems was individually adjusted so that each subject would achieve 80% correct performance. Last, because the mathematical test conditions were always paired with monetary reward, we cannot exclude the possibility that methylphenidate enhanced the saliency of the monetary reward rather than of the mathematical test itself. A study that assesses the effects of methylphenidate on a mathematical task that is not remunerated would enable research to address this issue. This is relevant because most of the behavioral strategies in ADHD use interventions that reinforce good behaviors (such as token reinforcers that can be exchanged for computer games, individual time with staff, special outings, and time in the playground, which in a way are similar to the use of money in this study).
In summary, these results provide evidence that methylphenidate’s effects on dopamine are sensitive to the conditions of its administration. Dopamine increases were larger when methylphenidate was given with a task that required cognitive performance that was remunerated than when it was given with a task that did not require performance and was not remunerated. They also show that methylphenidate enhanced the motivational saliency of the cognitive task, as evidenced by the perception of the task as more interesting, exciting, and motivating. The significant association between methylphenidate-induced dopamine increases and the perception of the task as interesting and motivating lead us to postulate that methylphenidate’s therapeutic effects may be secondary to its ability to enhance stimuli-induced dopamine increases, thus making them more motivationally salient and thereby improving performance. This brings to light the possibility that methylphenidate’s improvement of performance may be secondary to its motivational effects, which would explain why stimulants improve performance of a boring task in normal healthy individuals and why unmedicated ADHD children perform properly when the task is salient
(40). These findings support educational strategies that make schoolwork more interesting as nonpharmacological interventions to treat ADHD.